Abstract
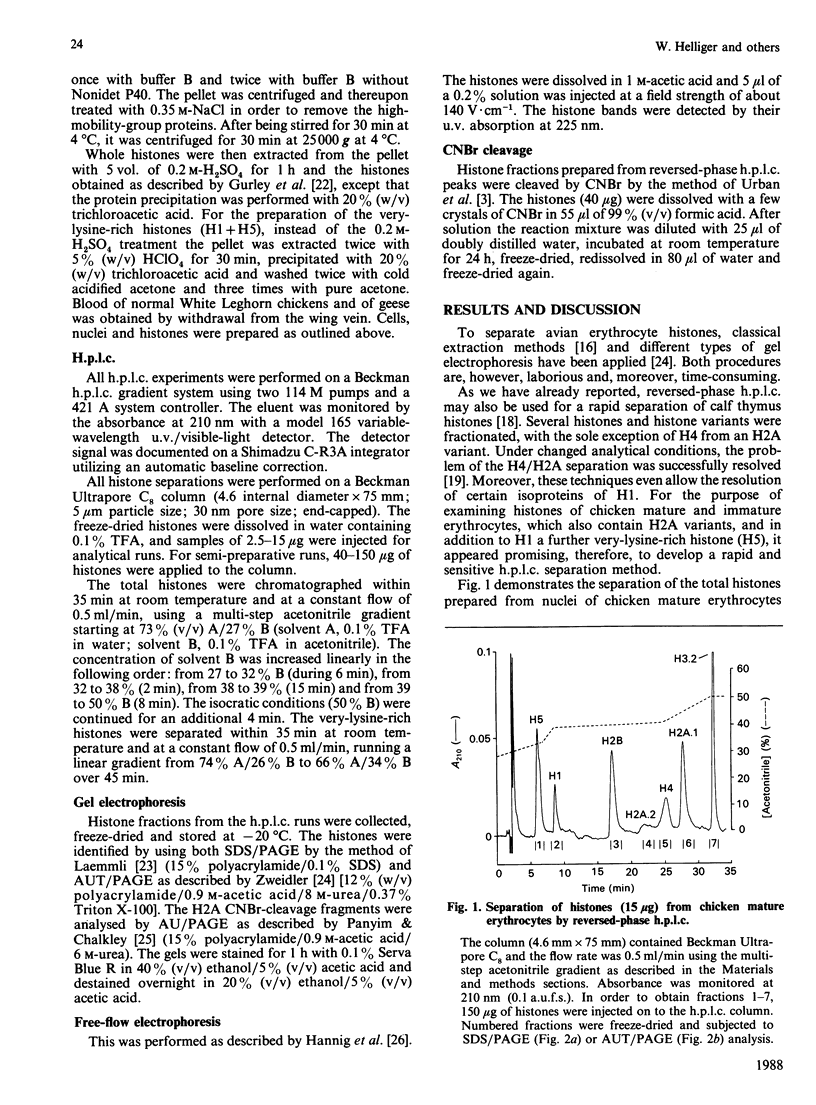
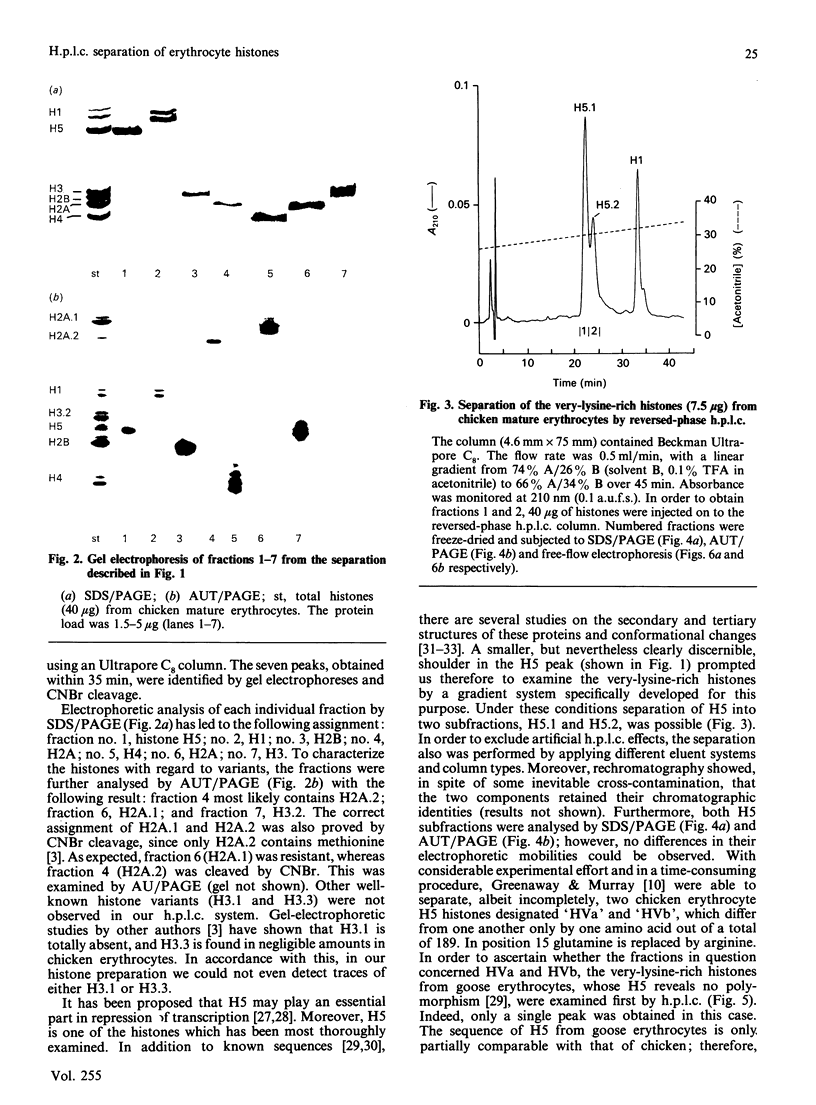
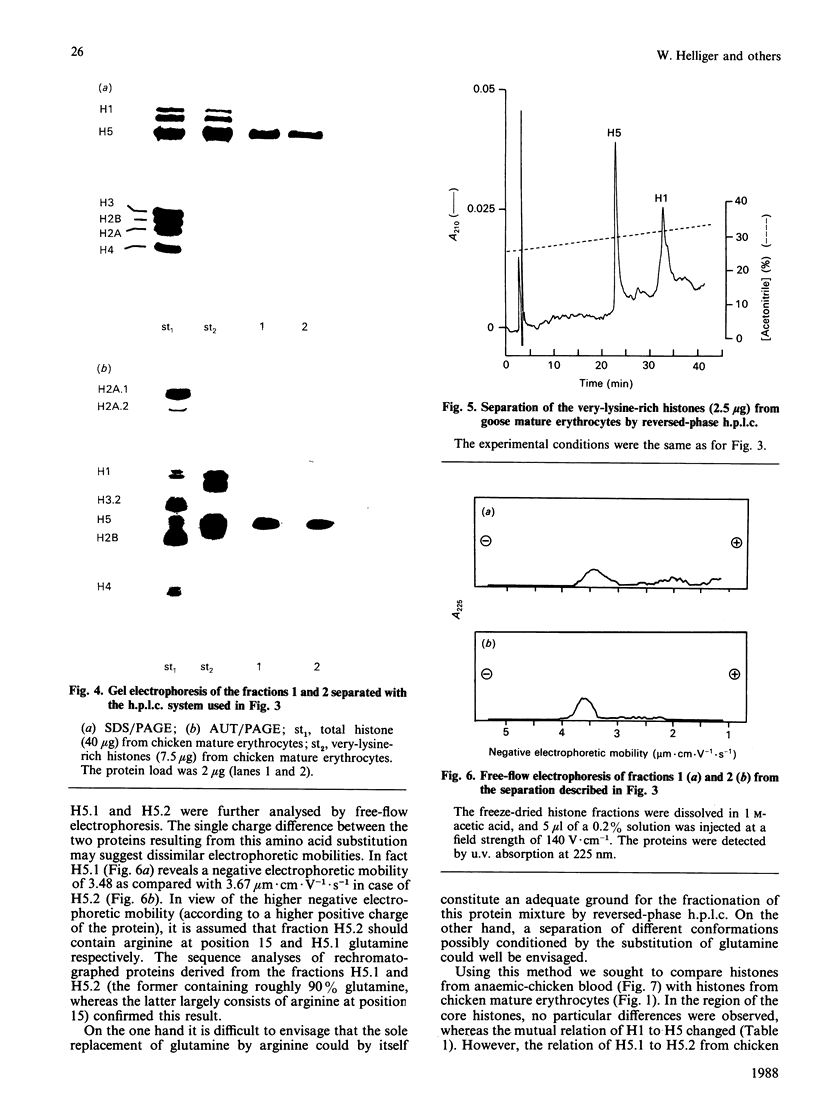
Total chicken erythrocyte histones were separated by reversed-phase h.p.l.c. using a multi-step acetonitrile gradient in a very short time (35 min). The proteins were eluted in the following order: H1, H5, H2B, H2A.2, H4, H2A.1 and H3.2. Applying a special gradient system adapted for the separation of very-lysine-rich histones, chicken erythrocyte H5 was resolved into two subfractions. Their electrophoretic mobilities were identical in both SDS and acetic acid/urea/Triton polyacrylamide-gel electrophoresis, but different in free-flow electrophoresis. Amino-acid-sequence analyses revealed that the two components only differ with respect to position 15, one having glutamine in that position and the other arginine. A separation of histones prepared from goose erythrocytes disclosed no H5 subfractionation. Furthermore, histones obtained from anaemic-chicken blood were analysed by the above-mentioned h.p.l.c. conditions. An alteration in the relation of H1 to H5 was detected, but no further differences in the number and quantity of the histones and histone variants were observed as compared with the corresponding proteins processed from normal-chicken blood.
Full text
PDF
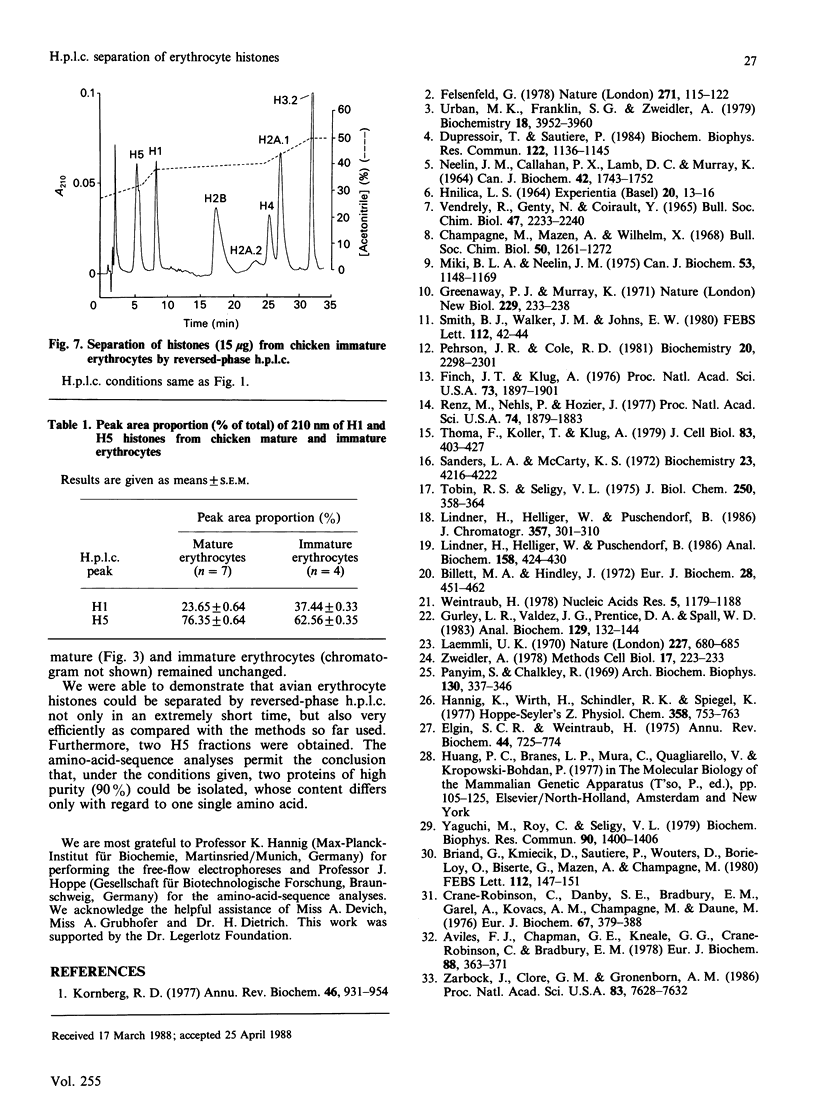
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviles F. J., Chapman G. E., Kneale G. G., Crane-Robinson C., Bradbury E. M. The conformation of histone H5. Isolation and characterisation of the globular segment. Eur J Biochem. 1978 Aug 1;88(2):363–371. doi: 10.1111/j.1432-1033.1978.tb12457.x. [DOI] [PubMed] [Google Scholar]
- Billett M. A., Hindley J. A study of the quantitative variation of histones, and their relationship to RNA synthesis, during erythropoiesis in the adult chicken. Eur J Biochem. 1972 Aug 4;28(4):451–462. doi: 10.1111/j.1432-1033.1972.tb01932.x. [DOI] [PubMed] [Google Scholar]
- Briand G., Kmiecik D., Sautiere P., Wouters D., Borie-Loy O., Biserte G., Mazen A., Champagne M. Chicken erythrocyte histone H5. IV. Sequence of the carboxy-termined half of the molecule (96 residues) and complete sequence. FEBS Lett. 1980 Apr 7;112(2):147–151. doi: 10.1016/0014-5793(80)80167-0. [DOI] [PubMed] [Google Scholar]
- Champagne M., Mazen A., Wilhelm X. Histones d'érythrocytes de poulets. I. Fractionnement des histones totales et isolement d'une histone spécifique. Bull Soc Chim Biol (Paris) 1968 Nov 5;50(7):1261–1272. [PubMed] [Google Scholar]
- Crane-Robinson C., Dancy S. E., Bradbury E. M., Garel A., Kovacs A. M., Champagne M., Daune M. Structural studies of chicken erythrocyte histone H5. Eur J Biochem. 1976 Aug 16;67(2):379–388. doi: 10.1111/j.1432-1033.1976.tb10702.x. [DOI] [PubMed] [Google Scholar]
- Dupressoir T., Sautiere P. Isolation and characterization of five subfractions of chicken erythrocyte histone H1. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1136–1145. doi: 10.1016/0006-291x(84)91210-5. [DOI] [PubMed] [Google Scholar]
- Elgin S. C., Weintraub H. Chromosomal proteins and chromatin structure. Annu Rev Biochem. 1975;44:725–774. doi: 10.1146/annurev.bi.44.070175.003453. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Klug A. Solenoidal model for superstructure in chromatin. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1897–1901. doi: 10.1073/pnas.73.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenaway P. J., Murray K. Heterogeneity and polymorphism in chicken erythrocyte histone fraction V. Nat New Biol. 1971 Feb 24;229(8):233–238. doi: 10.1038/newbio229233a0. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Valdez J. G., Prentice D. A., Spall W. D. Histone fractionation by high-performance liquid chromatography. Anal Biochem. 1983 Feb 15;129(1):132–144. doi: 10.1016/0003-2697(83)90061-1. [DOI] [PubMed] [Google Scholar]
- Hannig K., Wirth H., Schindler R. K., Spiegel K. Free-flow electrophoresis. III. An analytical version for a rapid, quantitative determination of electrophoretic parameters. Hoppe Seylers Z Physiol Chem. 1977 Jul;358(7):753–763. doi: 10.1515/bchm2.1977.358.2.753. [DOI] [PubMed] [Google Scholar]
- Hnilica L. S. The specificity of histones in chicken erythrocytes. Experientia. 1964 Jan 15;20(1):13–14. doi: 10.1007/BF02146014. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. doi: 10.1146/annurev.bi.46.070177.004435. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindner H., Helliger W., Puschendorf B. Histone separation by high-performance liquid chromatography on C4 reverse-phase columns. Anal Biochem. 1986 Nov 1;158(2):424–430. doi: 10.1016/0003-2697(86)90570-1. [DOI] [PubMed] [Google Scholar]
- Miki B. L., Neelin J. M. The histones of rainbow trout erythrocytes include an erythrocyte-specific histone. Can J Biochem. 1975 Nov;53(11):1158–1169. doi: 10.1139/o75-161. [DOI] [PubMed] [Google Scholar]
- NEELIN J. M., CALLAHAN P. X., LAMB D. C., MURRAY K. THE HISTONES OF CHICKEN ERYTHROCYTE NUCLEI. Can J Biochem. 1964 Dec;42:1743–1752. doi: 10.1139/o64-185. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Pehrson J. R., Cole R. D. Bovine H10 histone subfractions contain an invariant sequence which matches histones H5 rather than H1. Biochemistry. 1981 Apr 14;20(8):2298–2301. doi: 10.1021/bi00511a035. [DOI] [PubMed] [Google Scholar]
- Renz M., Nehls P., Hozier J. Involvement of histone H1 in the organization of the chromosome fiber. Proc Natl Acad Sci U S A. 1977 May;74(5):1879–1883. doi: 10.1073/pnas.74.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders L. A., McCarty K. S. Isolation and purification of histones from avian erythrocytes. Biochemistry. 1972 Nov 7;11(23):4216–4222. doi: 10.1021/bi00773a004. [DOI] [PubMed] [Google Scholar]
- Smith B. J., Walker J. M., Johns E. W. Structural homology between a mammalian H1(0) subfraction and avian erythrocyte-specific histone H5. FEBS Lett. 1980 Mar 24;112(1):42–44. doi: 10.1016/0014-5793(80)80122-0. [DOI] [PubMed] [Google Scholar]
- Thoma F., Koller T., Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J Cell Biol. 1979 Nov;83(2 Pt 1):403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin R. S., Seligy V. L. Characterization of chromatin-bound erythrocyte histone V (f2c). Synthesis, acetylation, and phosphorylation. J Biol Chem. 1975 Jan 25;250(2):358–364. [PubMed] [Google Scholar]
- Urban M. K., Franklin S. G., Zweidler A. Isolation and characterization of the histone variants in chicken erythrocytes. Biochemistry. 1979 Sep 4;18(18):3952–3960. doi: 10.1021/bi00585a017. [DOI] [PubMed] [Google Scholar]
- Weintraub H. The nucleosome repeat length increases during erythropoiesis in the chick. Nucleic Acids Res. 1978 Apr;5(4):1179–1188. doi: 10.1093/nar/5.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi M., Roy C., Seligy V. L. Complete amino acid sequence of goose erythrocyte H5 histone and the homology between H1 and H5 histones. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1400–1406. doi: 10.1016/0006-291x(79)91191-4. [DOI] [PubMed] [Google Scholar]
- Zarbock J., Clore G. M., Gronenborn A. M. Nuclear magnetic resonance study of the globular domain of chicken histone H5: resonance assignment and secondary structure. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7628–7632. doi: 10.1073/pnas.83.20.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweidler A. Resolution of histones by polyacrylamide gel electrophoresis in presence of nonionic detergents. Methods Cell Biol. 1978;17:223–233. [PubMed] [Google Scholar]