Abstract
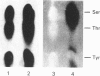
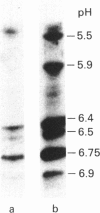
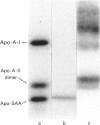
Monokine-induced hepatic secretion of serum amyloid A protein (apo-SAA), an acute-phase reactant, is followed by rapid association with high-density lipoprotein (HDL) in plasma. Plasma clearance of apo-SAA is more rapid than any of the other HDL apolipoproteins. It has been shown that, of the acute-phase HDL3 apolipoproteins, apo-SAA preferentially associates with neutrophil membranes. HDL apolipoproteins have been shown to activate protein kinase C in endothelial cells. We therefore investigated potential phosphorylation of HDL3 apolipoproteins by protein kinase C. Apo-SAA was the only apolipoprotein phosphorylated (Km = 12 mM). Phosphorylation of the apo-SAA-containing HDL3 particle was selective for the more basic isoforms of apo-SAA (pI 7.0, 7.4, 7.5 and 8.0), with more acidic isoforms being phosphorylated when delipidated acute-phase apolipoproteins were used as substrate. However, phosphorylation was not in itself responsible for the establishment of the apo-SAA isoforms.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bausserman L. L., Herbert P. N., McAdam K. P. Heterogeneity of human serum amyloid A proteins. J Exp Med. 1980 Sep 1;152(3):641–656. doi: 10.1084/jem.152.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Amyloid protein SAA is associated with high density lipoprotein from human serum. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4025–4028. doi: 10.1073/pnas.74.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Coetzee G. A., Strachan A. F., van der Westhuyzen D. R., Hoppe H. C., Jeenah M. S., de Beer F. C. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. J Biol Chem. 1986 Jul 25;261(21):9644–9651. [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Darbon J. M., Tournier J. F., Tauber J. P., Bayard F. Possible role of protein phosphorylation in the mitogenic effect of high density lipoproteins on cultured vascular endothelial cells. J Biol Chem. 1986 Jun 15;261(17):8002–8008. [PubMed] [Google Scholar]
- De Beer F. C., Mallya R. K., Fagan E. A., Lanham J. G., Hughes G. R., Pepys M. B. Serum amyloid-A protein concentration in inflammatory diseases and its relationship to the incidence of reactive systemic amyloidosis. Lancet. 1982 Jul 31;2(8292):231–234. doi: 10.1016/s0140-6736(82)90321-x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Godenir N. L., Jeenah M. S., Coetzee G. A., Van der Westhuyzen D. R., Strachan A. F., De Beer F. C. Standardisation of the quantitation of serum amyloid A protein (SAA) in human serum. J Immunol Methods. 1985 Nov 7;83(2):217–225. doi: 10.1016/0022-1759(85)90243-1. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Husebekk A., Skogen B., Husby G., Marhaug G. Transformation of amyloid precursor SAA to protein AA and incorporation in amyloid fibrils in vivo. Scand J Immunol. 1985 Mar;21(3):283–287. doi: 10.1111/j.1365-3083.1985.tb01431.x. [DOI] [PubMed] [Google Scholar]
- Itaya K., Ui M. A new micromethod for the colorimetric determination of inorganic phosphate. Clin Chim Acta. 1966 Sep;14(3):361–366. doi: 10.1016/0009-8981(66)90114-8. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Andersson R. G., Wise B. C., Mackerlova L., Salomonsson I., Brackett N. L., Katoh N., Shoji M., Wrenn R. W. Calcium-dependent protein kinase: widespread occurrence in various tissues and phyla of the animal kingdom and comparison of effects of phospholipid, calmodulin, and trifluoperazine. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7039–7043. doi: 10.1073/pnas.77.12.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Parmelee D. C., Titani K., Ericsson L. H., Eriksen N., Benditt E. P., Walsh K. A. Amino acid sequence of amyloid-related apoprotein (apoSAA1) from human high-density lipoprotein. Biochemistry. 1982 Jul 6;21(14):3298–3303. doi: 10.1021/bi00257a008. [DOI] [PubMed] [Google Scholar]
- Scanu A. Forms of human serum high density lipoprotein protein. J Lipid Res. 1966 Mar;7(2):295–306. [PubMed] [Google Scholar]
- Shephard E. G., de Beer F. C., de Beer M. C., Jeenah M. S., Coetzee G. A., van der Westhuyzen D. R. Neutrophil association and degradation of normal and acute-phase high-density lipoprotein 3. Biochem J. 1987 Dec 15;248(3):919–926. doi: 10.1042/bj2480919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan A. F., de Beer F. C., van der Westhuyzen D. R., Coetzee G. A. Identification of three isoform patterns of human serum amyloid A protein. Biochem J. 1988 Feb 15;250(1):203–207. doi: 10.1042/bj2500203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979 May 25;254(10):3692–3695. [PubMed] [Google Scholar]
- Wooten M. W., Vandenplas M., Nel A. E. Rapid purification of protein kinase C from rat brain. A novel method employing protamine-agarose affinity column chromatography. Eur J Biochem. 1987 Apr 15;164(2):461–467. doi: 10.1111/j.1432-1033.1987.tb11079.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Tanabe K., Taguchi Y. N., Nishizawa M., Takahashi T., Matsukage A. Chick embryo DNA polymerase beta. Purified enzyme consists of a single Mr = 40,000 polypeptide. J Biol Chem. 1980 Oct 25;255(20):9942–9948. [PubMed] [Google Scholar]