Abstract
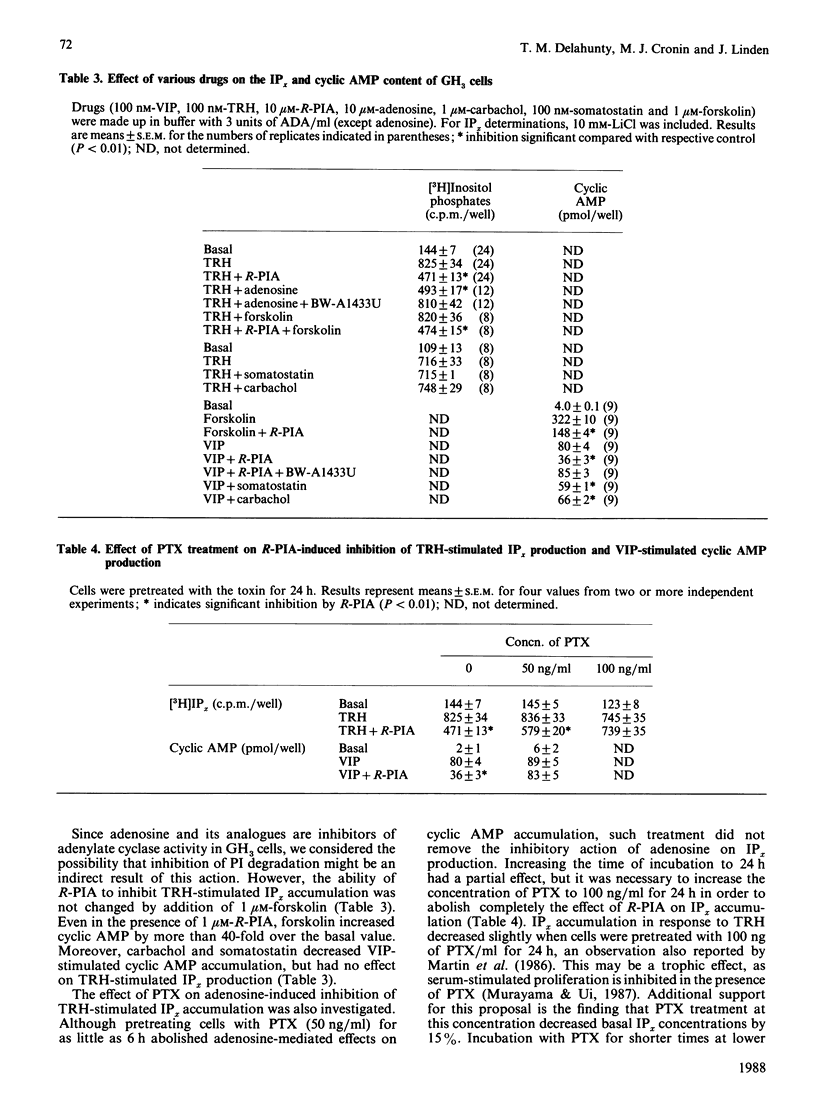
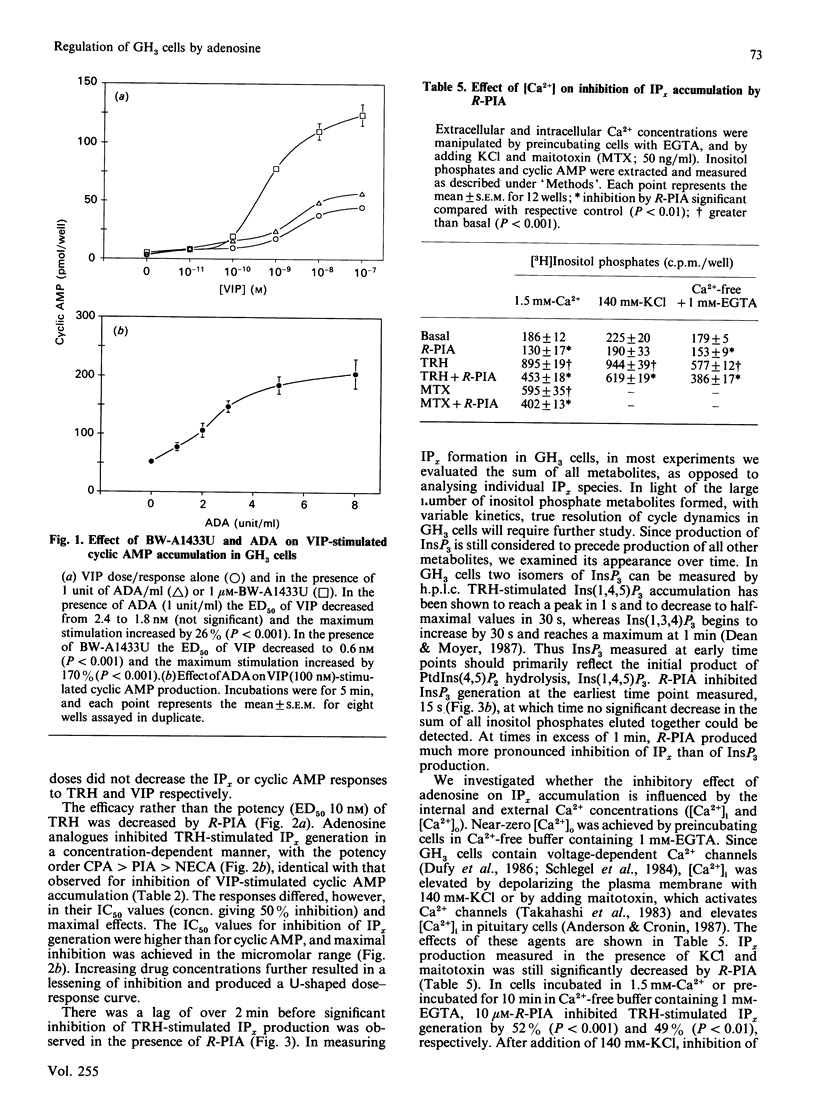
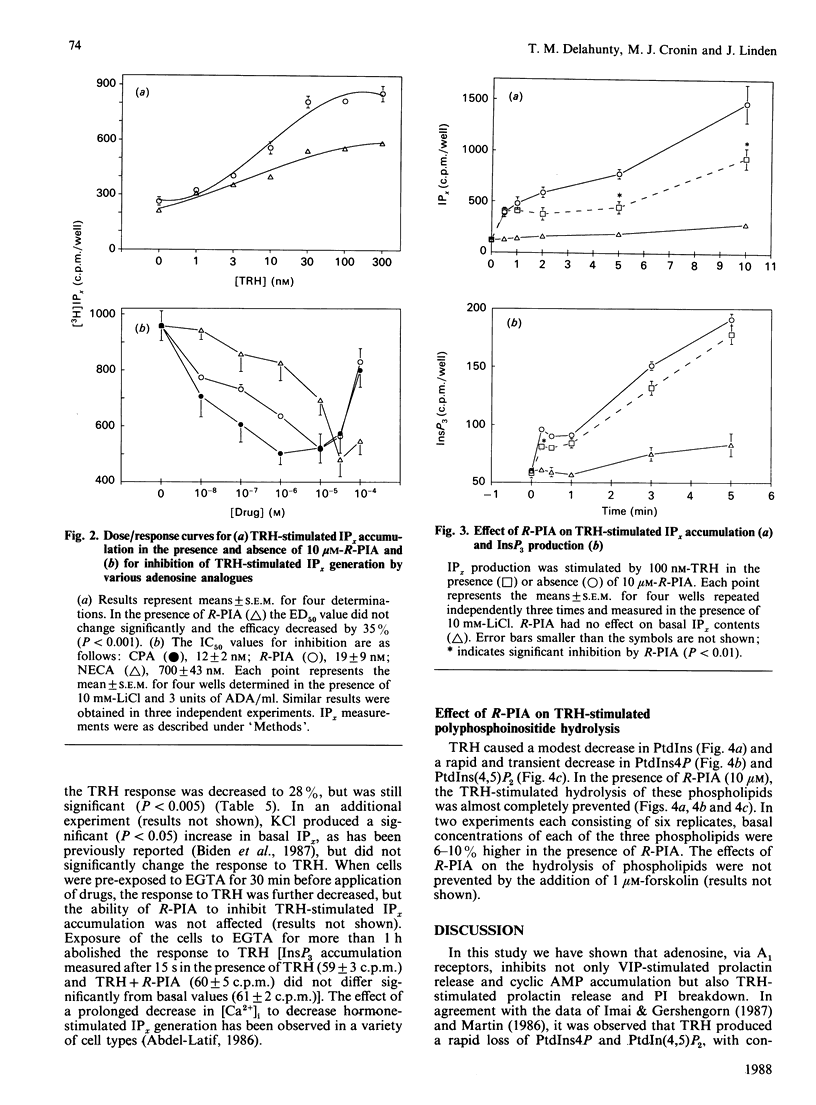
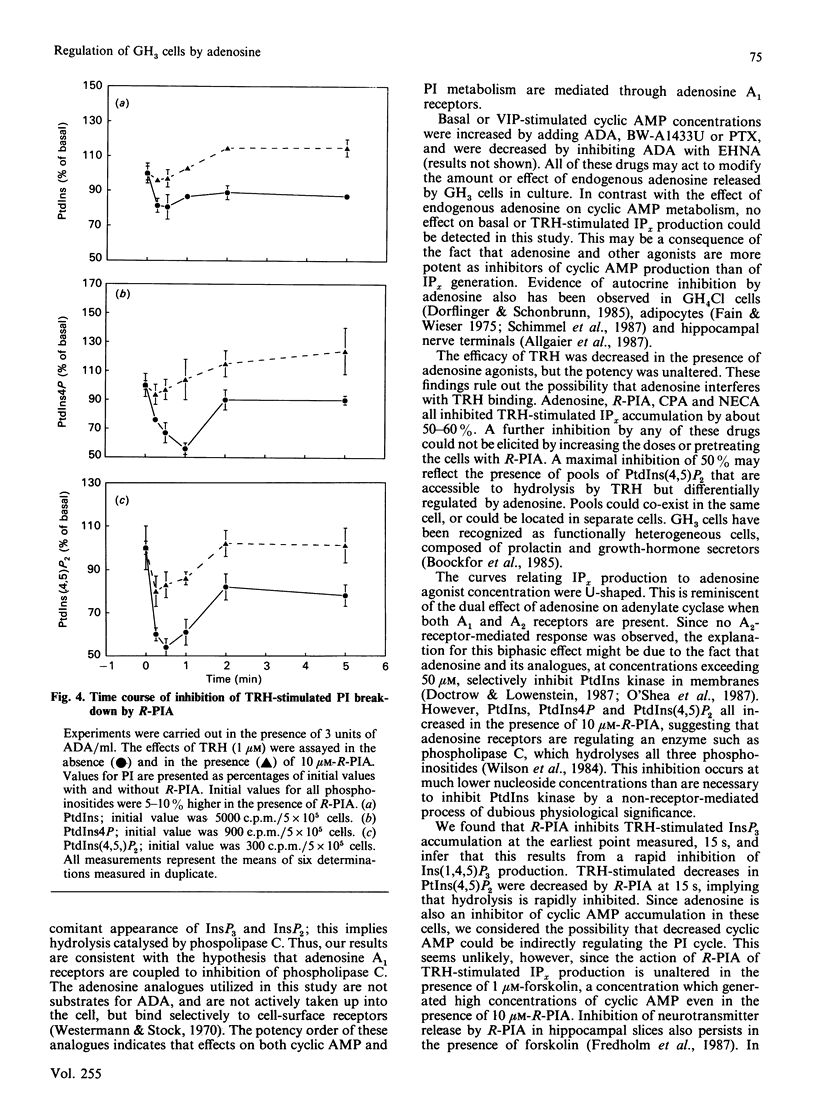
We examined the mechanism by which adenosine inhibits prolactin secretion from GH3 cells, a rat pituitary tumour line. Prolactin release is enhanced by vasoactive intestinal peptide (VIP), which increases cyclic AMP, and by thyrotropin-releasing hormone (TRH), which increases inositol phosphates (IPx). Analogues of adenosine decreased prolactin release, VIP-stimulated cyclic AMP accumulation and TRH-stimulated inositol phospholipid hydrolysis and IPx generation. Inhibition of InsP3 production by R-N6-phenylisopropyladenosine (R-PIA) was rapid (15 s) and was not affected by the addition of forskolin or the removal of external Ca2+. Addition of adenosine deaminase or the potent adenosine-receptor antagonist, BW-A1433U, enhanced the accumulation of cyclic AMP by VIP, indicating that endogenously produced adenosine tonically inhibits adenylate cyclase. The potency order of adenosine analogues for inhibition of cyclic AMP and IPx responses (measured in the presence of adenosine deaminase) was N6-cyclopentyladenosine greater than R-PIA greater than 5'-N-ethylcarboxamidoadenosine. This rank order indicates that inhibitions of both cyclic AMP and InsP3 production are mediated by adenosine A1 receptors. Responses to R-PIA were blocked by BW-A1433U (1 microM) or by pretreatment of cells with pertussis toxin. A greater amount of toxin was required to eliminate the effect of R-PIA on inositol phosphate than on cyclic AMP accumulation. These data indicate that adenosine, in addition to inhibiting cyclic AMP accumulation, decreases IPx production in GH3 cells, possibly by directly inhibiting phosphoinositide hydrolysis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
- Allgaier C., Hertting G., Kügelgen O. V. The adenosine receptor-mediated inhibition of noradrenaline release possibly involves an N-protein and is increased by alpha 2-autoreceptor blockade. Br J Pharmacol. 1987 Feb;90(2):403–412. doi: 10.1111/j.1476-5381.1987.tb08970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. M., Yasumoto T., Cronin M. J. Intracellular free calcium in rat anterior pituitary cells monitored by fura-2. Life Sci. 1987 Jul 27;41(4):519–526. doi: 10.1016/0024-3205(87)90230-x. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A., Winslow J. W., Peralta E. G., Peterson G. L., Schimerlik M. I., Capon D. J., Ramachandran J. An M2 muscarinic receptor subtype coupled to both adenylyl cyclase and phosphoinositide turnover. Science. 1987 Oct 30;238(4827):672–675. doi: 10.1126/science.2823384. [DOI] [PubMed] [Google Scholar]
- Baudry M., Evans J., Lynch G. Excitatory amino acids inhibit stimulation of phosphatidylinositol metabolism by aminergic agonists in hippocampus. Nature. 1986 Jan 23;319(6051):329–331. doi: 10.1038/319329a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Peter-Riesch B., Schlegel W., Wollheim C. B. Ca2+-mediated generation of inositol 1,4,5-triphosphate and inositol 1,3,4,5-tetrakisphosphate in pancreatic islets. Studies with K+, glucose, and carbamylcholine. J Biol Chem. 1987 Mar 15;262(8):3567–3571. [PubMed] [Google Scholar]
- Boockfor F. R., Hoeffler J. P., Frawley L. S. Cultures of GH3 cells are functionally heterogeneous: thyrotropin-releasing hormone, estradiol and cortisol cause reciprocal shifts in the proportions of growth hormone and prolactin secretors. Endocrinology. 1985 Jul;117(1):418–420. doi: 10.1210/endo-117-1-418. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Rubin R. P. Guanine nucleotide regulation of phospholipase C activity in permeabilized rabbit neutrophils. Inhibition by pertussis toxin and sensitization to submicromolar calcium concentrations. Biochem J. 1986 Oct 1;239(1):97–102. doi: 10.1042/bj2390097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker G., Terasaki W. L., Price M. G. Gammaflow: a completely automated radioimmunoassay system. Science. 1976 Oct 15;194(4262):270–276. doi: 10.1126/science.184530. [DOI] [PubMed] [Google Scholar]
- Canonico P. L., Jarvis W. D., Judd A. M., MacLeod R. M. Dopamine does not attenuate phosphoinositide hydrolysis in rat anterior pituitary cells. J Endocrinol. 1986 Sep;110(3):389–393. doi: 10.1677/joe.0.1100389. [DOI] [PubMed] [Google Scholar]
- Clemo H. F., Bourassa A., Linden J., Belardinelli L. Antagonism of the effects of adenosine and hypoxia on atrioventricular conduction time by two novel alkylxanthines: correlation with binding to adenosine A1 receptors. J Pharmacol Exp Ther. 1987 Aug;242(2):478–484. [PubMed] [Google Scholar]
- Dean N. M., Moyer J. D. Separation of multiple isomers of inositol phosphates formed in GH3 cells. Biochem J. 1987 Mar 1;242(2):361–366. doi: 10.1042/bj2420361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doctrow S. R., Lowenstein J. M. Inhibition of phosphatidylinositol kinase in vascular smooth muscle membranes by adenosine and related compounds. Biochem Pharmacol. 1987 Jul 15;36(14):2255–2262. doi: 10.1016/0006-2952(87)90588-0. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Prestwich S. A. Pertussis toxin reverses adenosine inhibition of neuronal glutamate release. Nature. 1985 Jul 11;316(6024):148–150. doi: 10.1038/316148a0. [DOI] [PubMed] [Google Scholar]
- Dorflinger L. J., Schonbrunn A. Adenosine inhibits prolactin and growth hormone secretion in a clonal pituitary cell line. Endocrinology. 1985 Dec;117(6):2330–2338. doi: 10.1210/endo-117-6-2330. [DOI] [PubMed] [Google Scholar]
- Drummond A. H., Bushfield M., Macphee C. H. Thyrotropin-releasing hormone-stimulated [3H]inositol metabolism in GH3 pituitary tumor cells. Studies with lithium. Mol Pharmacol. 1984 Mar;25(2):201–208. [PubMed] [Google Scholar]
- Dufy B., MacDermott A., Barker J. L. Rundown of GH3 cell K+ conductance response to TRH following patch recording can be obviated with GH3 cell extract. Biochem Biophys Res Commun. 1986 May 29;137(1):388–396. doi: 10.1016/0006-291x(86)91222-2. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T. V., Worth T. S., Olsson R. A. Adenosine analogs mediating depressant effects on synaptic transmission in rat hippocampus: structure-activity relationships for the N6 subregion. Naunyn Schmiedebergs Arch Pharmacol. 1986 Sep;334(1):77–85. doi: 10.1007/BF00498743. [DOI] [PubMed] [Google Scholar]
- Enjalbert A., Sladeczek F., Guillon G., Bertrand P., Shu C., Epelbaum J., Garcia-Sainz A., Jard S., Lombard C., Kordon C. Angiotensin II and dopamine modulate both cAMP and inositol phosphate productions in anterior pituitary cells. Involvement in prolactin secretion. J Biol Chem. 1986 Mar 25;261(9):4071–4075. [PubMed] [Google Scholar]
- Fain J. N., Wieser P. B. Effects of adenosine deaminase on cyclic adenosine monophosphate accumulation, lipolysis, and glucose metabolism of fat cells. J Biol Chem. 1975 Feb 10;250(3):1027–1034. [PubMed] [Google Scholar]
- Gourdji D., Bataille D., Vauclin N., Grouselle D., Rosselin G., Tixier-Vidal A. Vasoactive intestinal peptide (VIP) stimulates prolactin (PRL) release and cAMP production in a rat pituitary cell line (GH3/B6). Additive effects of VIP and TRH on PRL release. FEBS Lett. 1979 Aug 1;104(1):165–168. doi: 10.1016/0014-5793(79)81107-2. [DOI] [PubMed] [Google Scholar]
- Hill S. J., Kendall D. A. Studies on the adenosine-receptor mediating the augmentation of histamine-induced inositol phospholipid hydrolysis in guinea-pig cerebral cortex. Br J Pharmacol. 1987 Jul;91(3):661–669. doi: 10.1111/j.1476-5381.1987.tb11260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth E. B., De la Cruz R. A., Daly J. W. Accumulations of inositol phosphates and cyclic AMP in brain slices: synergistic interactions of histamine and 2-chloroadenosine. Eur J Pharmacol. 1986 Mar 11;122(1):45–50. doi: 10.1016/0014-2999(86)90156-1. [DOI] [PubMed] [Google Scholar]
- Imai A., Gershengorn M. C. Measurement of lipid turnover in response to thyrotropin-releasing hormone. Methods Enzymol. 1987;141:100–101. doi: 10.1016/0076-6879(87)41059-8. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. The enzymology of stimulated inositol lipid turnover. Cell Calcium. 1982 Oct;3(4-5):295–309. doi: 10.1016/0143-4160(82)90018-5. [DOI] [PubMed] [Google Scholar]
- Kendall D. A., Hill S. J. Adenosine inhibition of histamine-stimulated inositol phospholipid hydrolysis in mouse cerebral cortex. J Neurochem. 1988 Feb;50(2):497–502. doi: 10.1111/j.1471-4159.1988.tb02939.x. [DOI] [PubMed] [Google Scholar]
- Lewis D. L., Weight F. F., Luini A. A guanine nucleotide-binding protein mediates the inhibition of voltage-dependent calcium current by somatostatin in a pituitary cell line. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9035–9039. doi: 10.1073/pnas.83.23.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J., Vogel S., Sperelakis N. Sensitivity of Ca-dependent slow action potentials to methacholine is induced by phosphodiesterase inhibitors in embryonic chick ventricles. J Pharmacol Exp Ther. 1982 Aug;222(2):383–388. [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C. J., Stone T. W. Adenosine reduces agonist-induced production of inositol phosphates in rat aorta. J Pharm Pharmacol. 1987 Dec;39(12):1010–1014. doi: 10.1111/j.2042-7158.1987.tb03149.x. [DOI] [PubMed] [Google Scholar]
- Martin T. F., Lucas D. O., Bajjalieh S. M., Kowalchyk J. A. Thyrotropin-releasing hormone activates a Ca2+-dependent polyphosphoinositide phosphodiesterase in permeable GH3 cells. GTP gamma S potentiation by a cholera and pertussis toxin-insensitive mechanism. J Biol Chem. 1986 Feb 25;261(6):2918–2927. [PubMed] [Google Scholar]
- Martin T. F. Measurement of phospholipid turnover in cultured hormone responsive pituitary cells. Methods Enzymol. 1986;124:424–442. doi: 10.1016/0076-6879(86)24033-1. [DOI] [PubMed] [Google Scholar]
- Martin T. F. Thyrotropin-releasing hormone rapidly activates the phosphodiester hydrolysis of polyphosphoinositides in GH3 pituitary cells. Evidence for the role of a polyphosphoinositide-specific phospholipase C in hormone action. J Biol Chem. 1983 Dec 25;258(24):14816–14822. [PubMed] [Google Scholar]
- Murayama T., Ui M. Loss of the inhibitory function of the guanine nucleotide regulatory component of adenylate cyclase due to its ADP ribosylation by islet-activating protein, pertussis toxin, in adipocyte membranes. J Biol Chem. 1983 Mar 10;258(5):3319–3326. [PubMed] [Google Scholar]
- Murayama T., Ui M. Possible involvement of a GTP-binding protein, the substrate of islet-activating protein, in receptor-mediated signaling responsible for cell proliferation. J Biol Chem. 1987 Sep 15;262(26):12463–12467. [PubMed] [Google Scholar]
- O'Shea J. J., Suárez-Quian C. A., Swank R. A., Klausner R. D. The inhibitory effect of cyclic AMP on phosphatidylinositol kinase is not mediated by the cAMP dependent protein kinase. Biochem Biophys Res Commun. 1987 Jul 31;146(2):561–567. doi: 10.1016/0006-291x(87)90565-1. [DOI] [PubMed] [Google Scholar]
- Petcoff D. W., Cooper D. M. Adenosine receptor agonists inhibit inositol phosphate accumulation in rat striatal slices. Eur J Pharmacol. 1987 Jun 4;137(2-3):269–271. doi: 10.1016/0014-2999(87)90234-2. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Wu P. H. The role of adenosine and its nucleotides in central synaptic transmission. Prog Neurobiol. 1981;16(3-4):187–239. doi: 10.1016/0301-0082(81)90014-9. [DOI] [PubMed] [Google Scholar]
- Sattin A., Rall T. W. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3', 5'-phosphate content of guinea pig cerebral cortex slices. Mol Pharmacol. 1970 Jan;6(1):13–23. [PubMed] [Google Scholar]
- Schimmel R. J., Elliott M. E., Dehmel V. C. Interactions between adenosine and alpha 1-adrenergic agonists in regulation of respiration in hamster brown adipocytes. Mol Pharmacol. 1987 Jul;32(1):26–33. [PubMed] [Google Scholar]
- Schlegel W., Wuarin F., Wollheim C. B., Zahnd G. R. Somatostatin lowers the cytosolic free Ca2+ concentration in clonal rat pituitary cells (GH3 cells). Cell Calcium. 1984 Jun;5(3):223–236. doi: 10.1016/0143-4160(84)90038-1. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Cox C. C., Snyderman R. Receptor-coupled activation of phosphoinositide-specific phospholipase C by an N protein. Science. 1986 Apr 4;232(4746):97–100. doi: 10.1126/science.3006254. [DOI] [PubMed] [Google Scholar]
- Sutton C. A., Martin T. F. Thyrotropin-releasing hormone (TRH) selectively and rapidly stimulates phosphatidylinositol turnover in GH pituitary cells: a possible second step of TRH action. Endocrinology. 1982 Apr;110(4):1273–1280. doi: 10.1210/endo-110-4-1273. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Tatsumi M., Ohizumi Y., Yasumoto T. Ca2+ channel activating function of maitotoxin, the most potent marine toxin known, in clonal rat pheochromocytoma cells. J Biol Chem. 1983 Sep 25;258(18):10944–10949. [PubMed] [Google Scholar]
- Williams M. Purine receptors in mammalian tissues: pharmacology and functional significance. Annu Rev Pharmacol Toxicol. 1987;27:315–345. doi: 10.1146/annurev.pa.27.040187.001531. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Bross T. E., Hofmann S. L., Majerus P. W. Hydrolysis of polyphosphoinositides by purified sheep seminal vesicle phospholipase C enzymes. J Biol Chem. 1984 Oct 10;259(19):11718–11724. [PubMed] [Google Scholar]
- Wojcikiewicz R. J., Kent P. A., Fain J. N. Evidence that thyrotropin-releasing hormone-induced increases in GTPase activity and phosphoinositide metabolism in GH3 cells are mediated by a guanine nucleotide-binding protein other than Gs or Gi. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1383–1389. doi: 10.1016/s0006-291x(86)80436-3. [DOI] [PubMed] [Google Scholar]
- Wolff J., Londos C., Cooper D. M. Adenosine receptors and the regulation of adenylate cyclase. Adv Cyclic Nucleotide Res. 1981;14:199–214. [PubMed] [Google Scholar]
- Yajima Y., Akita Y., Saito T. Pertussis toxin blocks the inhibitory effects of somatostatin on cAMP-dependent vasoactive intestinal peptide and cAMP-independent thyrotropin releasing hormone-stimulated prolactin secretion of GH3 cells. J Biol Chem. 1986 Feb 25;261(6):2684–2689. [PubMed] [Google Scholar]
- Yatani A., Codina J., Brown A. M., Birnbaumer L. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science. 1987 Jan 9;235(4785):207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]
- Zysk J. R., Pobiner B. F., Hewlett E. L., Garrison J. C., Cronin M. J. Pertussis toxin mediates ADP-ribosylation of pituitary membrane proteins. Endocr Res. 1986;12(2):157–170. doi: 10.1080/07435808609035435. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


