Abstract
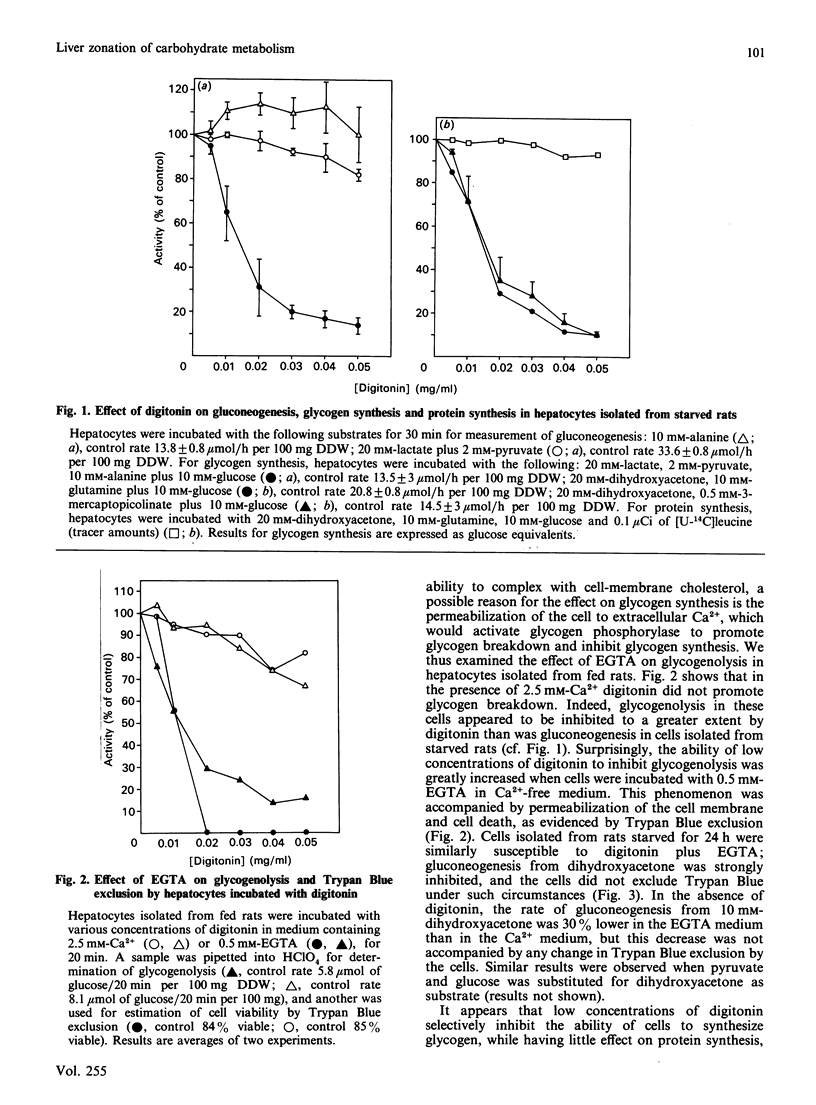
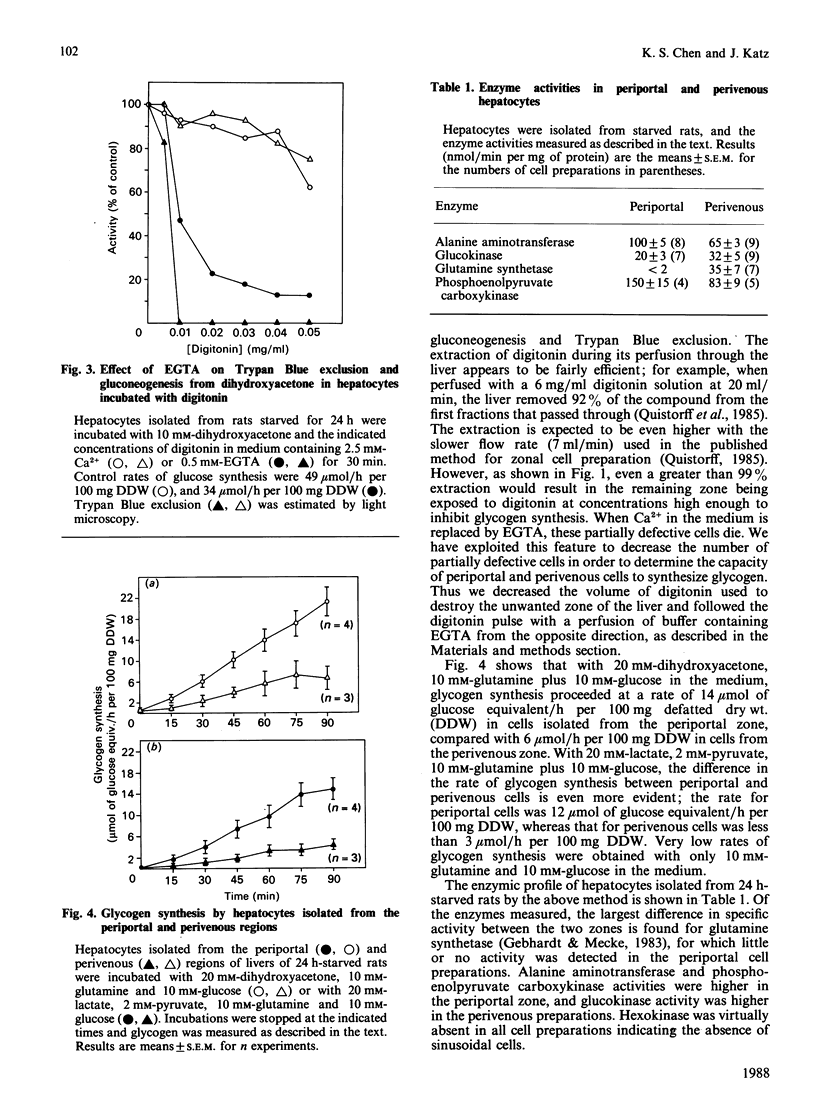
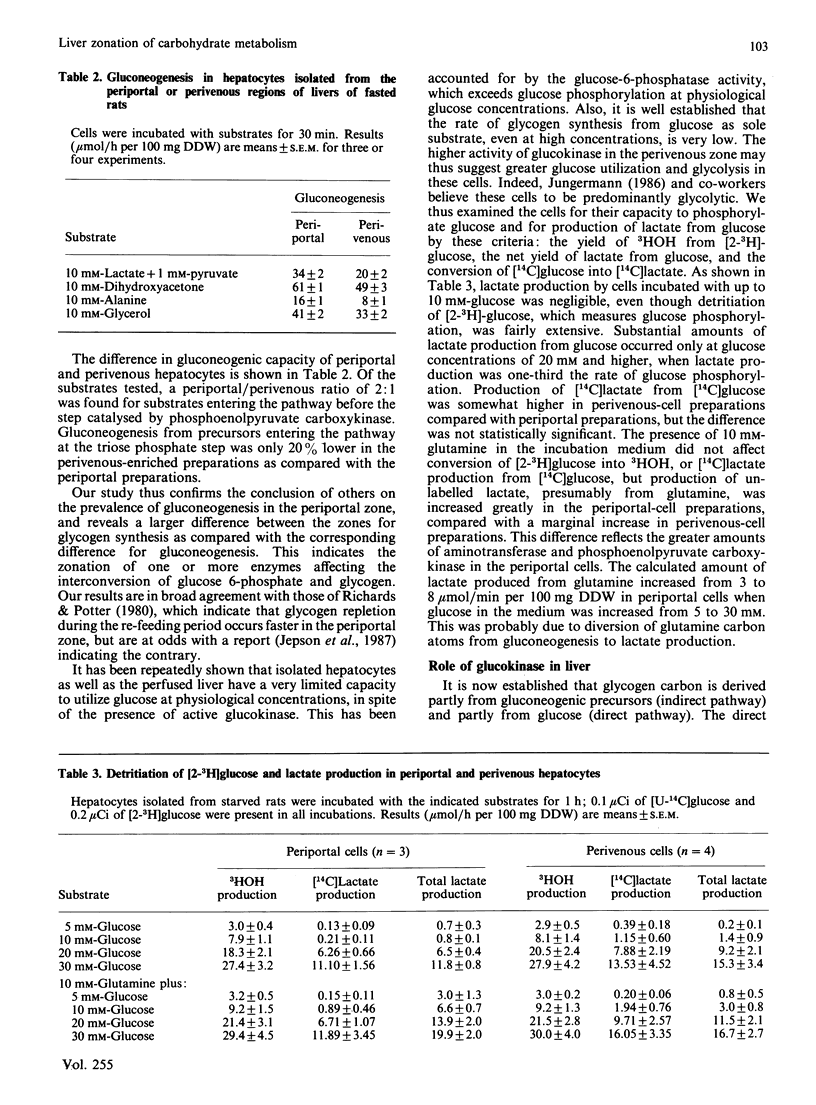
We have investigated the cause of defective glycogen synthesis in hepatocyte preparations enriched with cells from the periportal or perivenous zones obtained by the methods of Lindros & Penttila [Biochem. J. (1985) 228, 757-760] and of Quistorff [Biochem. J. (1985) 229, 221-226]. A modified procedure which yields hepatocytes capable of consistent rates of glycogen synthesis is described, and the rates of glucose and glycogen syntheses and of glycolysis in hepatocytes from the two zones are compared. Glycogen synthesis in cells was greatly impaired by very low concentrations (0.01-0.05 mg/ml) of digitonin, which had little effect on glucose and protein syntheses and Trypan Blue exclusion. Cells exposed to such low concentrations of digitonin lose all their synthetic capacity and ability to exclude Trypan Blue when incubated with EGTA, which does not affect cells not exposed to digitonin. With a modified procedure based on this phenomenon, our study reveals that hepatocyte preparations enriched with cells from the periportal zone synthesized glucose from lactate and alanine at rates twice those by cells from the perivenous zone, whereas the rate of glycogen synthesis from C3 precursors in periportal cells was 4 times that in the perivenous preparations. With substrates entering the pathway at the triose phosphate level, gluconeogenesis in periportal-cell preparations was 20% higher, and glycogen synthesis was twice that in perivenous preparations. Glycolysis was studied by the formation of 3HOH from [2-3H]glucose, the yield of lactate, and the conversion of [14C]glucose into [14C]lactate. In cell preparations from both zones glycolysis by all criteria was negligible at 10 mM-glucose, but was substantial at higher concentrations. However, there was no difference between the zones. We confirm that the capacities for glucose and glycogen syntheses in periportal cells are higher than in perivenous cells, but that at physiological glucose concentrations there is negligible glycolysis in liver parenchyma in both zones. The metabolic pattern in the perivenous cells is not glycolytic.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartels H., Vogt B., Jungermann K. Glycogen synthesis from pyruvate in the periportal and from glucose in the perivenous zone in perfused livers from fasted rats. FEBS Lett. 1987 Sep 14;221(2):277–283. doi: 10.1016/0014-5793(87)80940-7. [DOI] [PubMed] [Google Scholar]
- Bentle L. A., Lardy H. A. Interaction of anions and divalent metal ions with phosphoenolpyruvate carboxykinase. J Biol Chem. 1976 May 25;251(10):2916–2921. [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. S., Lardy H. A. Multiple requirements for glycogen synthesis by hepatocytes isolated from fasted rats. J Biol Chem. 1985 Nov 25;260(27):14683–14688. [PubMed] [Google Scholar]
- Davis M. A., Williams P. E., Cherrington A. D. Net hepatic lactate balance following mixed meal feeding in the four-day fasted conscious dog. Metabolism. 1987 Sep;36(9):856–862. doi: 10.1016/0026-0495(87)90094-1. [DOI] [PubMed] [Google Scholar]
- Gebhardt R., Mecke D. Heterogeneous distribution of glutamine synthetase among rat liver parenchymal cells in situ and in primary culture. EMBO J. 1983;2(4):567–570. doi: 10.1002/j.1460-2075.1983.tb01464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L., Hers H. G. Utile and futile cycles in the liver. Biochem Biophys Res Commun. 1974 Jun 4;58(3):540–548. doi: 10.1016/s0006-291x(74)80454-7. [DOI] [PubMed] [Google Scholar]
- Jungermann K. Functional heterogeneity of periportal and perivenous hepatocytes. Enzyme. 1986;35(3):161–180. doi: 10.1159/000469338. [DOI] [PubMed] [Google Scholar]
- Katz J., Golden S., Wals P. A. Stimulation of hepatic glycogen synthesis by amino acids. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3433–3437. doi: 10.1073/pnas.73.10.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Wals P. A., Golden S., Rognstad R. Recycling of glucose by rat hepatocytes. Eur J Biochem. 1975 Dec 1;60(1):91–101. doi: 10.1111/j.1432-1033.1975.tb20979.x. [DOI] [PubMed] [Google Scholar]
- Lindros K. O., Penttilä K. E. Digitonin-collagenase perfusion for efficient separation of periportal or perivenous hepatocytes. Biochem J. 1985 Jun 15;228(3):757–760. doi: 10.1042/bj2280757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima F., Katz J. Effect of mercaptopicolinic acid and of transaminase inhibitors on glycogen synthesis by rat hepatocytes. Biochem Biophys Res Commun. 1979 Mar 15;87(1):155–162. doi: 10.1016/0006-291x(79)91660-7. [DOI] [PubMed] [Google Scholar]
- Quistorff B., Dich J., Grunnet N. Periportal and perivenous hepatocytes retain their zonal characteristics in primary culture. Biochem Biophys Res Commun. 1986 Sep 30;139(3):1055–1061. doi: 10.1016/s0006-291x(86)80284-4. [DOI] [PubMed] [Google Scholar]
- Quistorff B. Gluconeogenesis in periportal and perivenous hepatocytes of rat liver, isolated by a new high-yield digitonin/collagenase perfusion technique. Biochem J. 1985 Jul 1;229(1):221–226. doi: 10.1042/bj2290221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistorff B., Grunnet N., Cornell N. W. Digitonin perfusion of rat liver. A new approach in the study of intra-acinar and intracellular compartmentation in the liver. Biochem J. 1985 Feb 15;226(1):289–297. doi: 10.1042/bj2260289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards W. L., Potter V. R. Scanning microdensitometry of glycogen zonation in the livers of rats adapted to a controlled feeding schedule and to 30, 60, or 90% casein diets. Am J Anat. 1980 Jan;157(1):71–85. doi: 10.1002/aja.1001570108. [DOI] [PubMed] [Google Scholar]
- Vorhaben J. E., Wong L., Campbell J. W. Assay for glutamine synthetase activity. Biochem J. 1973 Dec;135(4):893–896. doi: 10.1042/bj1350893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara H., Thurman R. G. Involvement of calmodulin-calcium complex in regulation of O2 uptake in regions of the liver lobule. Am J Physiol. 1987 Sep;253(3 Pt 1):G383–G389. doi: 10.1152/ajpgi.1987.253.3.G383. [DOI] [PubMed] [Google Scholar]


