Abstract
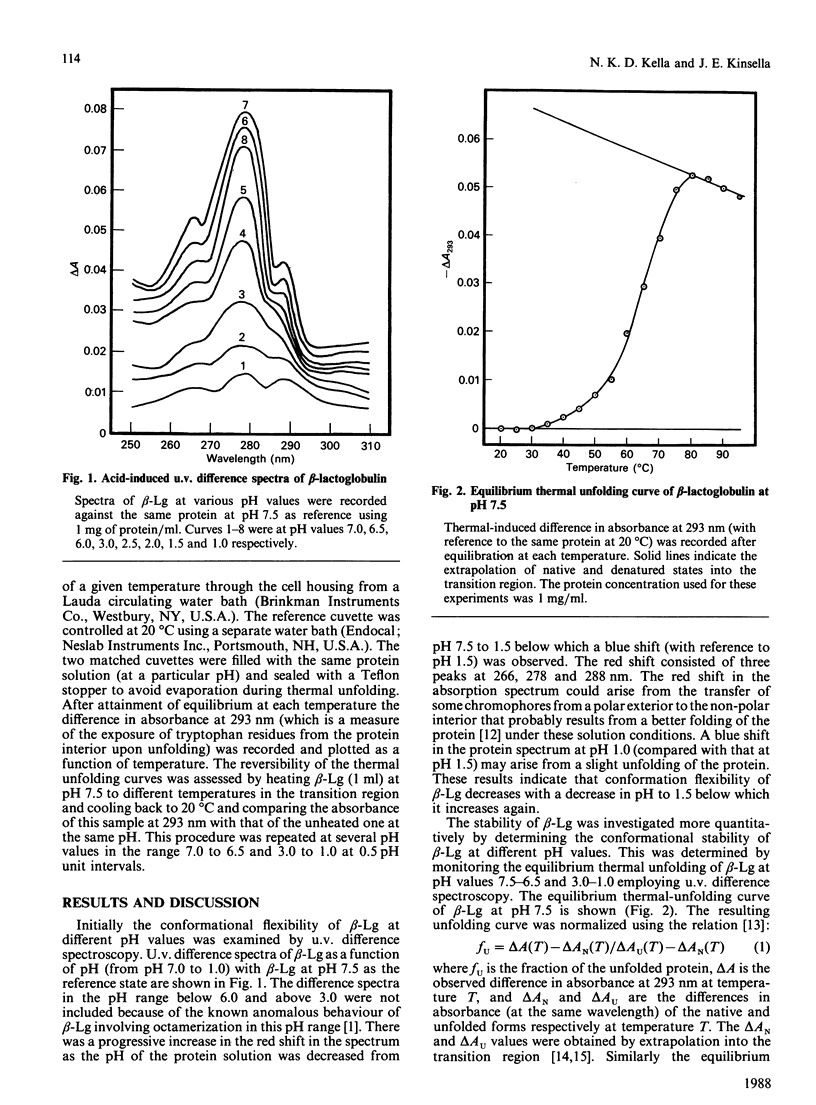
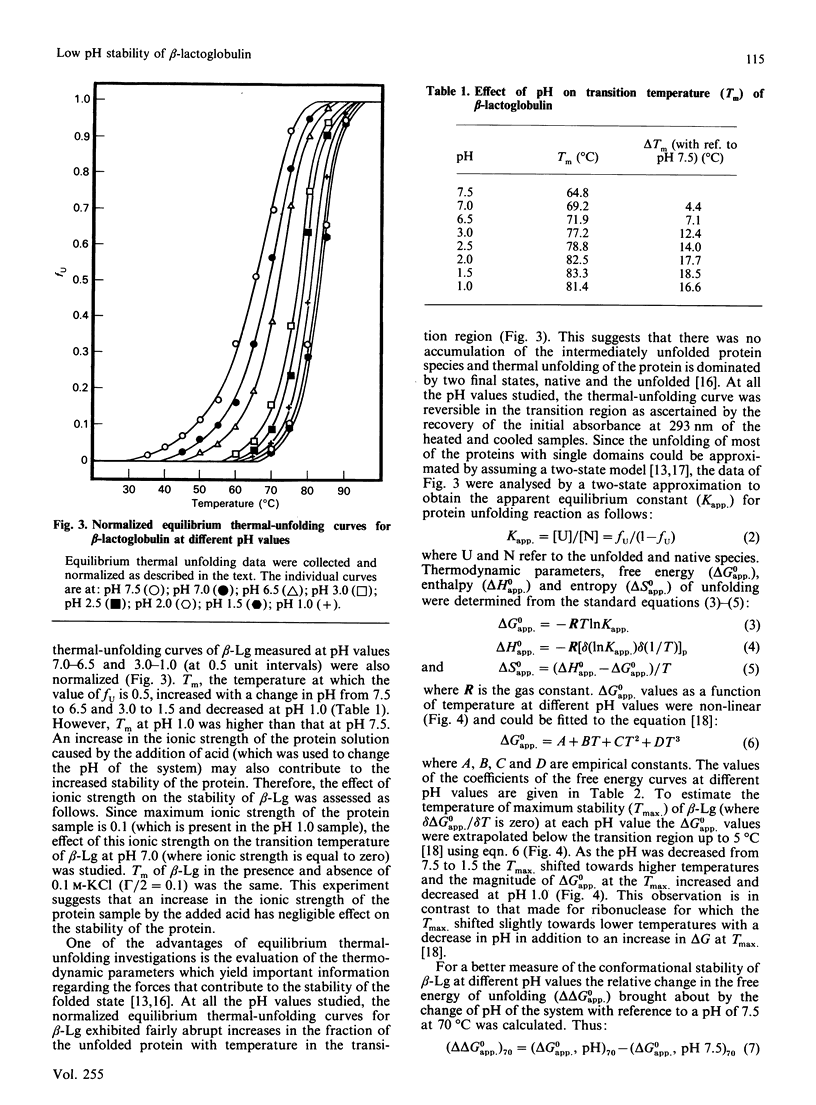
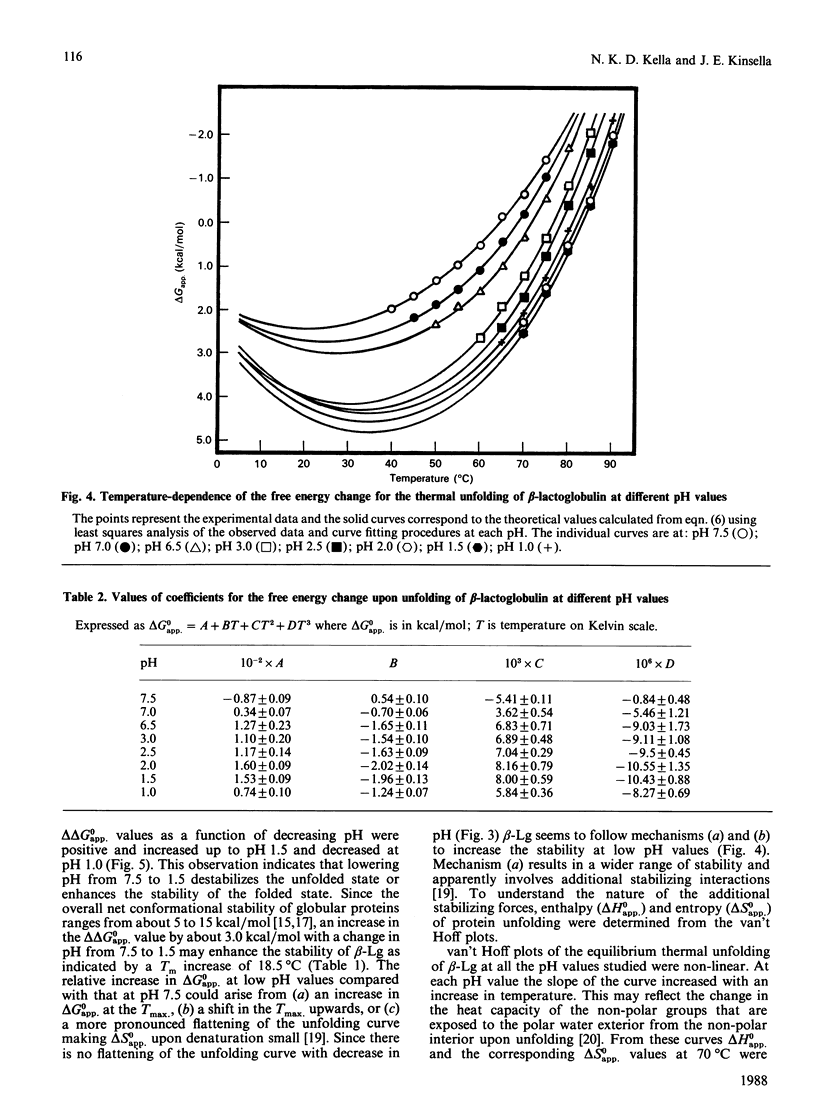
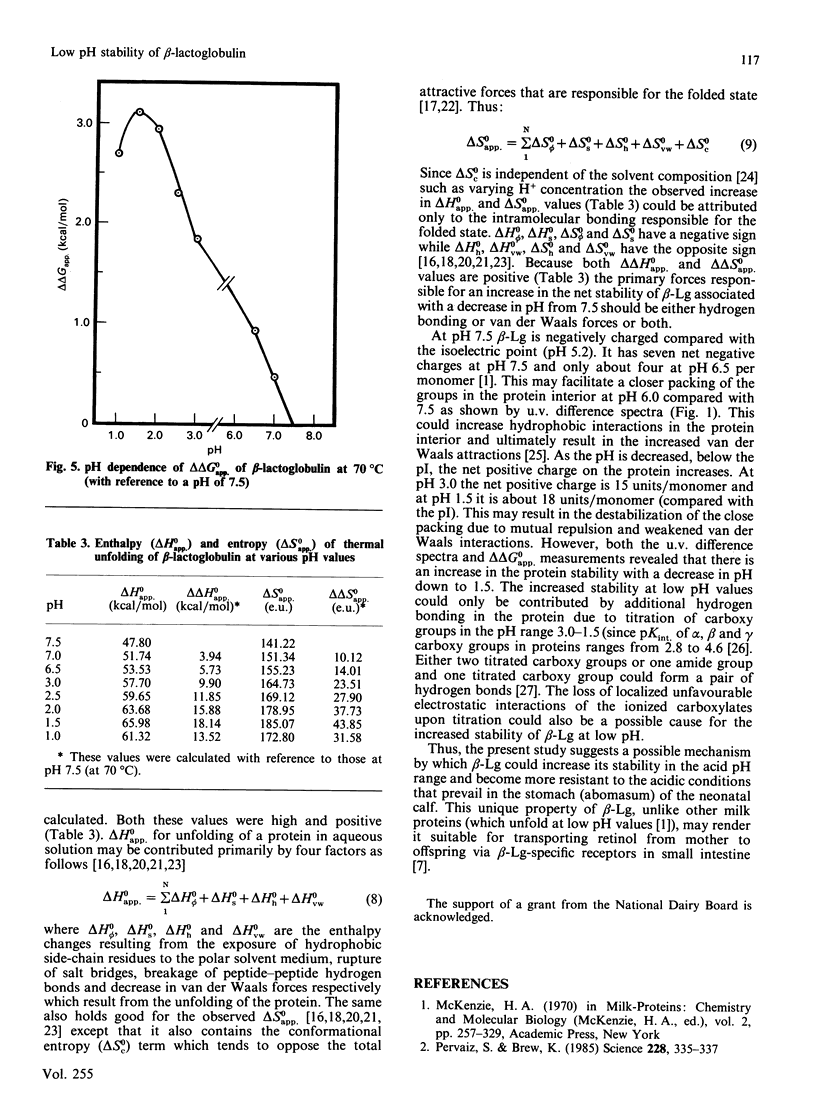
The thermodynamic stability of beta-lactoglobulin (beta-Lg) was studied at acidic and near-neutral pH values using equilibrium thermal-unfolding measurements. Transition temperature increased with a decrease in pH from 7.5 to 6.5 and 3.0 to 1.5, suggesting an increase in the net protein stability. Determination of the change in free energy of unfolding and extrapolation into the nontransition region revealed that beta-Lg increases its stability by increasing the magnitude of the change in free energy of unfolding at the temperature of maximum stability, as well as by increasing the temperature of maximum stability. The relative difference in the change in free energy of unfolding at 70 degrees C (with a reference pH of 7.5) was positive and its magnitude increased with a decrease in pH from 7.0 to 1.5 van't Hoff plots of thermal unfolding of beta-Lg at all pH values studied were non-linear and the measured changes in the enthalpy and entropy of unfolding for beta-Lg were high and positive. The relative magnitude of change of both enthalpy and entropy at 70 degrees C (compared with pH 7.5) increased with a decrease in pH up to 1.5. A possible mechanism for the increased stability of beta-Lg at low pH is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASCHAFFENBURG R., DREWRY J. Improved method for the preparation of crystalline beta-lactoglobulin and alpha-lactalbumin from cow's milk. Biochem J. 1957 Feb;65(2):273–277. doi: 10.1042/bj0650273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandts J. F., Hunt L. The thermodynamics of protein denaturation. 3. The denaturation of ribonuclease in water and in aqueous urea and aqueous ethanol mixtures. J Am Chem Soc. 1967 Sep 13;89(19):4826–4838. doi: 10.1021/ja00995a002. [DOI] [PubMed] [Google Scholar]
- Byler D. M., Susi H., Farrell H. M., Jr Laser-Raman spectra, sulfhydryl groups, and conformation of the cystine linkages of beta-lactoglobulin. Biopolymers. 1983 Dec;22(12):2507–2511. doi: 10.1002/bip.360221204. [DOI] [PubMed] [Google Scholar]
- Fugate R. D., Song P. S. Spectroscopic characterization of beta-lactoglobulin-retinol complex. Biochim Biophys Acta. 1980 Sep 23;625(1):28–42. doi: 10.1016/0005-2795(80)90105-1. [DOI] [PubMed] [Google Scholar]
- Gekko K., Timasheff S. N. Thermodynamic and kinetic examination of protein stabilization by glycerol. Biochemistry. 1981 Aug 4;20(16):4677–4686. doi: 10.1021/bi00519a024. [DOI] [PubMed] [Google Scholar]
- Green D. W., Aschaffenburg R., Camerman A., Coppola J. C., Dunnill P., Simmons R. M., Komorowski E. S., Sawyer L., Turner E. M., Woods K. F. Structure of bovine beta-lactoglobulin at 6A resolution. J Mol Biol. 1979 Jun 25;131(2):375–397. doi: 10.1016/0022-2836(79)90082-2. [DOI] [PubMed] [Google Scholar]
- Hermans J., Jr Methods for the study of reversible denaturation of proteins and interpretation of data. Methods Biochem Anal. 1965;13:81–111. [PubMed] [Google Scholar]
- Nojima H., Ikai A., Oshima T., Noda H. Reversible thermal unfolding of thermostable phosphoglycerate kinase. Thermostability associated with mean zero enthalpy change. J Mol Biol. 1977 Nov 5;116(3):429–442. doi: 10.1016/0022-2836(77)90078-x. [DOI] [PubMed] [Google Scholar]
- Pace C. N. The stability of globular proteins. CRC Crit Rev Biochem. 1975 May;3(1):1–43. doi: 10.3109/10409237509102551. [DOI] [PubMed] [Google Scholar]
- Papiz M. Z., Sawyer L., Eliopoulos E. E., North A. C., Findlay J. B., Sivaprasadarao R., Jones T. A., Newcomer M. E., Kraulis P. J. The structure of beta-lactoglobulin and its similarity to plasma retinol-binding protein. 1986 Nov 27-Dec 3Nature. 324(6095):383–385. doi: 10.1038/324383a0. [DOI] [PubMed] [Google Scholar]
- Pervaiz S., Brew K. Homology of beta-lactoglobulin, serum retinol-binding protein, and protein HC. Science. 1985 Apr 19;228(4697):335–337. doi: 10.1126/science.2580349. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Subramanian S. Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry. 1981 May 26;20(11):3096–3102. doi: 10.1021/bi00514a017. [DOI] [PubMed] [Google Scholar]
- Timasheff S. N., Mescanti L., Basch J. J., Townend R. Conformational transitions of bovine beta-lactoglobulins A, B, and C. J Biol Chem. 1966 Jun 10;241(11):2496–2501. [PubMed] [Google Scholar]


