Abstract
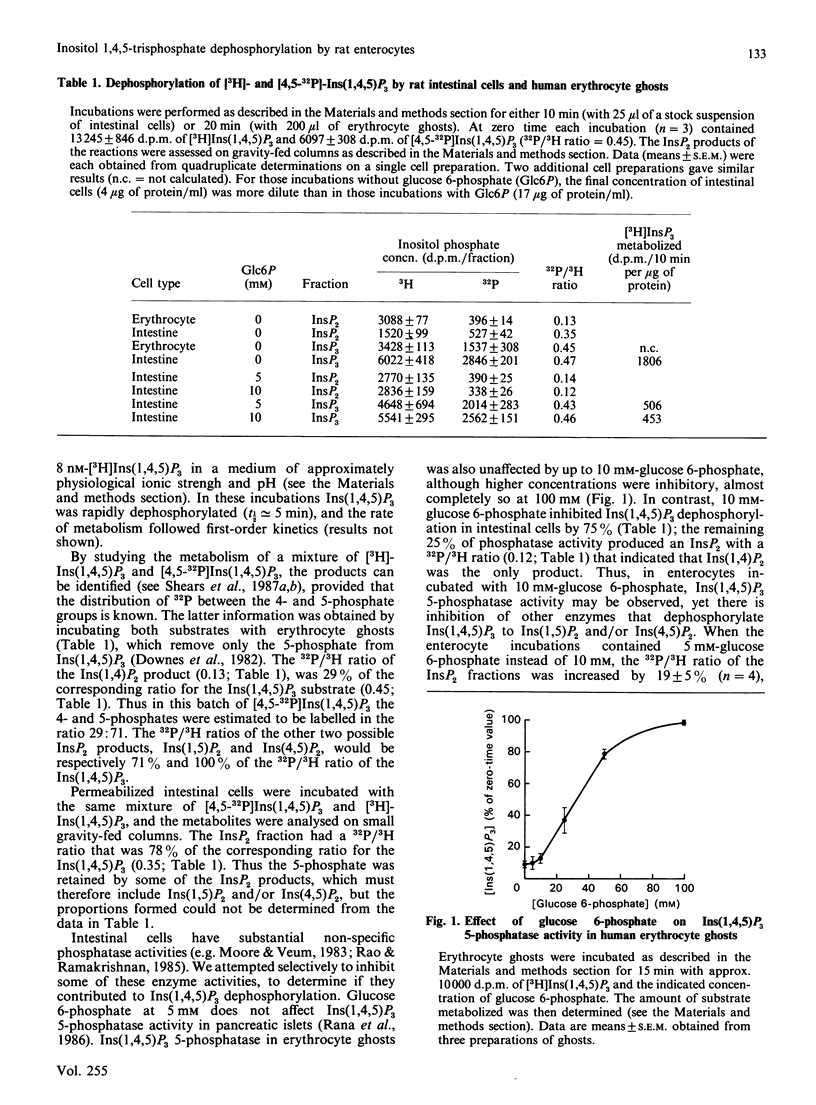
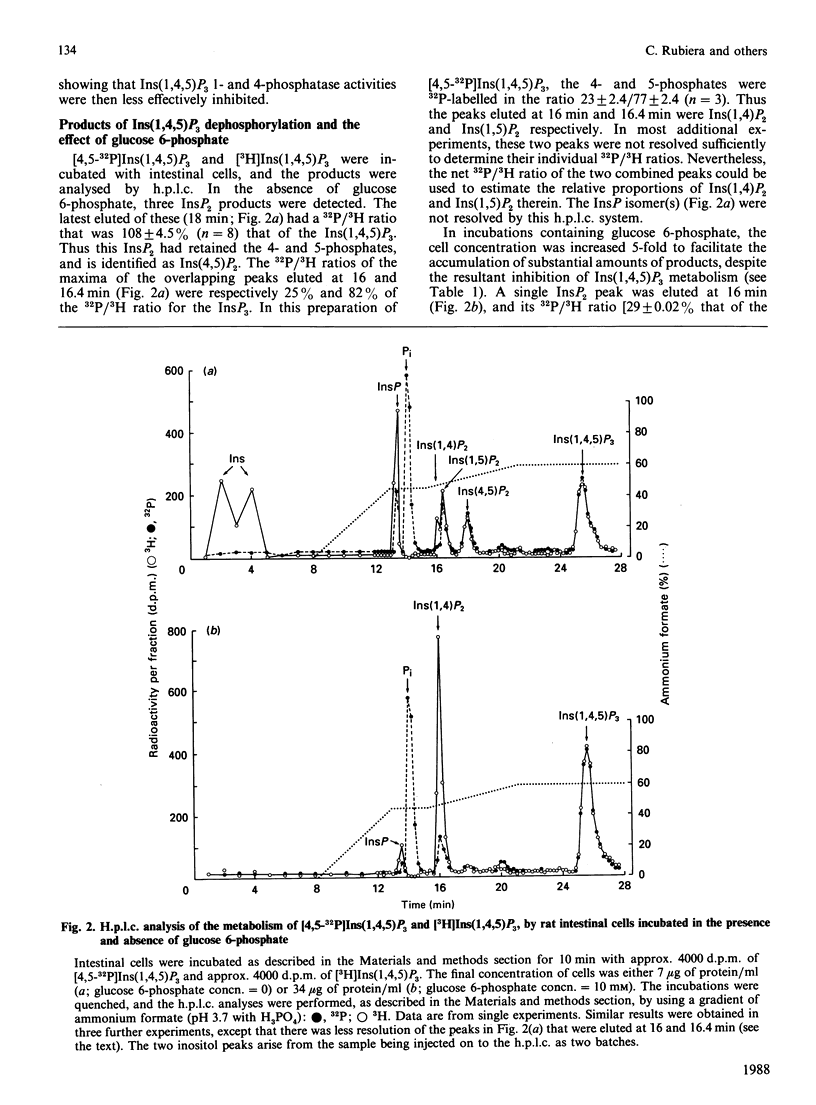
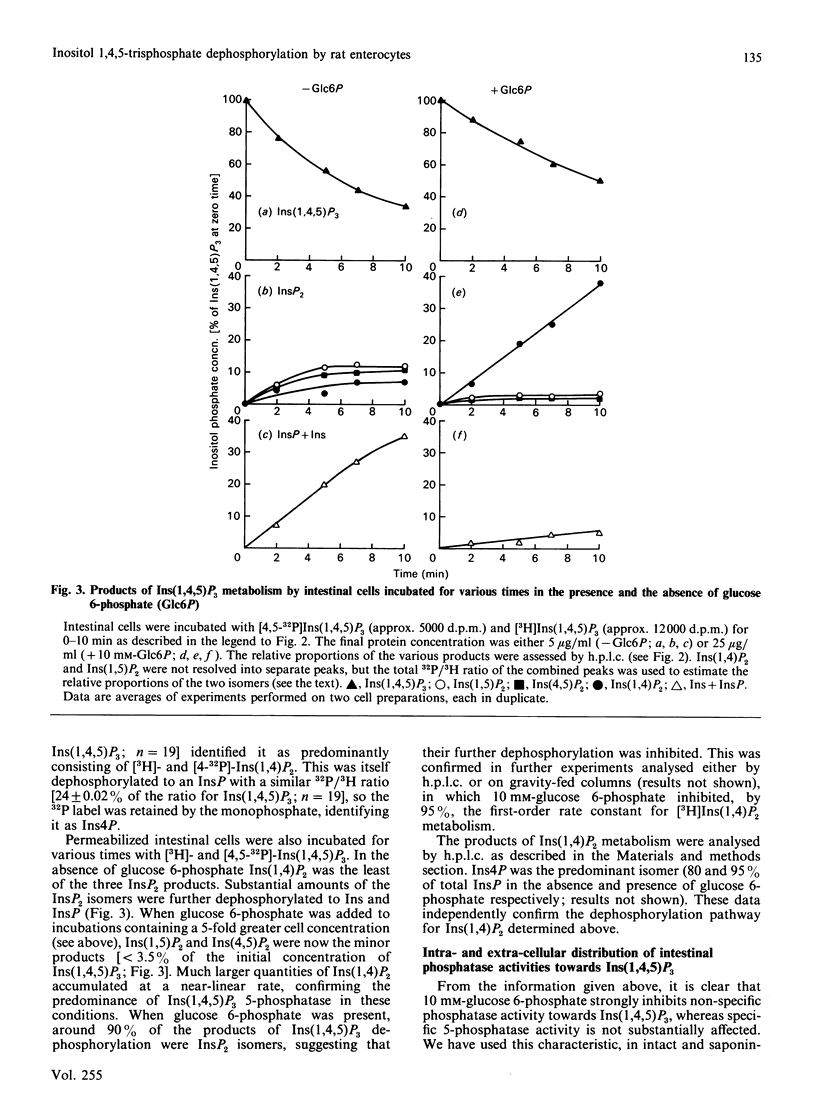
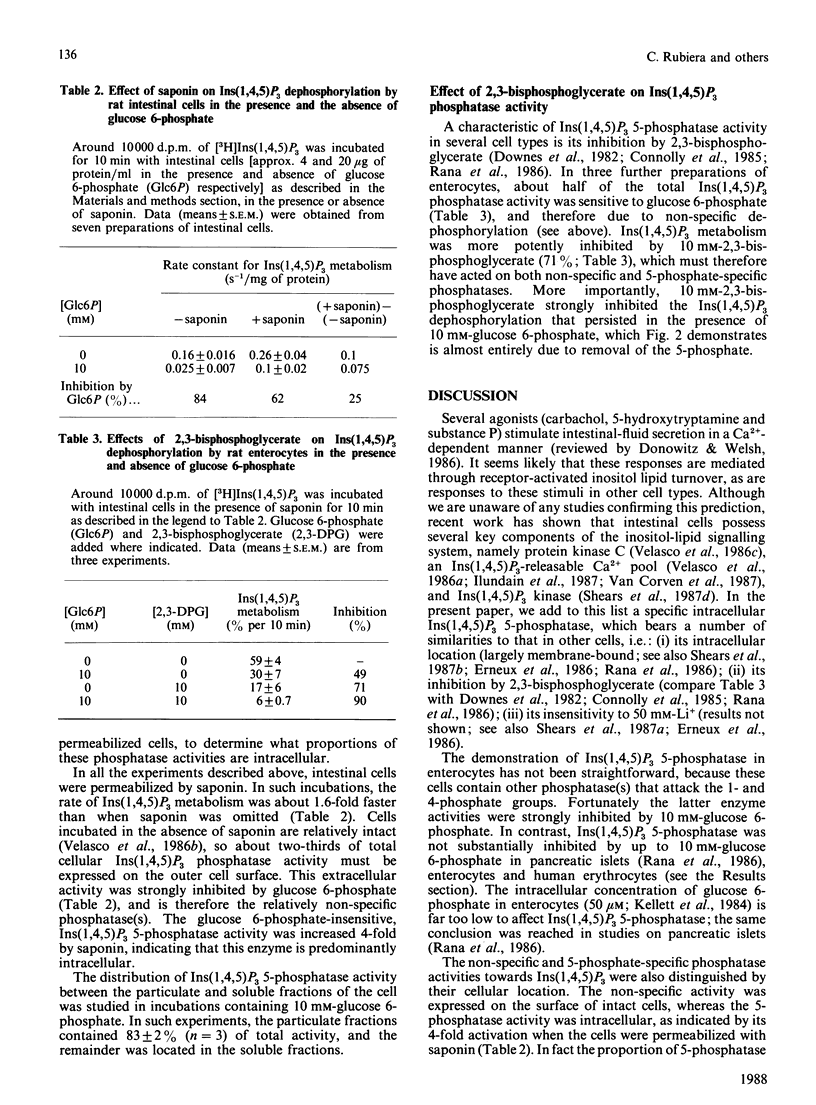
We studied the dephosphorylation of Ins(1,4,5)P3 (inositol 1,4,5-trisphosphate) by permeabilized rat intestinal epithelial cells incubated in a medium resembling intracellular ionic strength and pH. Saponin-permeabilized cells rapidly dephosphorylated Ins(1,4,5)P3 to a mixture of three InsP2 (inositol bisphosphate) isomers, namely Ins(1,4)P2, Ins(1,5)P2 and Ins(4,5)P2. These products were identified by h.p.l.c. analysis after dephosphorylation of both 3H- and 32P-labelled Ins(1,4,5)P3. Ins(1,4)P2 accumulated to about half of the concentration attained by Ins(1,5)P2 and Ins(4,5)P2. Ins(1,4,5)P3 dephosphorylation was inhibited, by up to 75%, by 10 mM-glucose 6-phosphate. In these conditions Ins(1,4)P2 became the predominant product, indicating that glucose 6-phosphate inhibited non-specific dephosphorylation of Ins(1,4,5)P3, at least at the 1- and 4-phosphate groups. Ins(1,4)P2 was further dephosphorylated, and the major InsP (inositol monophosphate) product was Ins4P. Most of the glucose 6-phosphate-inhibitable Ins(1,4,5)P3 phosphatase activity was exposed on the cell surface. The glucose 6-phosphate-insensitive Ins(1,4,5)P3 5-phosphatase activity was not detected until the cells were permeabilized with saponin. This intracellular 5-phosphatase activity was: (i) predominantly associated with the particulate portion of the cell; (ii) strongly inhibited by 10 mM-2,3-bisphosphoglycerate; (iii) insensitive to 50 mM-Li+. Therefore the Ins(1,4,5)P3 5-phosphatase activity in enterocytes appears similar to the 5-phosphatase that has been characterized in a number of cell types.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown D. M., Stewart J. C. The structure of triphosphoinositide from beef brain. Biochim Biophys Acta. 1966 Dec 7;125(3):413–421. doi: 10.1016/0005-2760(66)90029-4. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Fabiato A., Leslie B. A., Putney J. W., Jr Calcium pools in saponin-permeabilized guinea pig hepatocytes. J Biol Chem. 1983 Dec 25;258(24):15336–15345. [PubMed] [Google Scholar]
- Clarke N. G., Dawson R. M. Alkaline O leads to N-transacylation. A new method for the quantitative deacylation of phospholipids. Biochem J. 1981 Apr 1;195(1):301–306. doi: 10.1042/bj1950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T. M., Bross T. E., Majerus P. W. Isolation of a phosphomonoesterase from human platelets that specifically hydrolyzes the 5-phosphate of inositol 1,4,5-trisphosphate. J Biol Chem. 1985 Jul 5;260(13):7868–7874. [PubMed] [Google Scholar]
- Dean N. M., Moyer J. D. Separation of multiple isomers of inositol phosphates formed in GH3 cells. Biochem J. 1987 Mar 1;242(2):361–366. doi: 10.1042/bj2420361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M., Welsh M. J. Ca2+ and cyclic AMP in regulation of intestinal Na, K, and Cl transport. Annu Rev Physiol. 1986;48:135–150. doi: 10.1146/annurev.ph.48.030186.001031. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erneux C., Delvaux A., Moreau C., Dumont J. E. Characterization of D-myo-inositol 1,4,5-trisphosphate phosphatase in rat brain. Biochem Biophys Res Commun. 1986 Jan 14;134(1):351–358. doi: 10.1016/0006-291x(86)90570-x. [DOI] [PubMed] [Google Scholar]
- Hawkins P. T., Stephens L., Downes C. P. Rapid formation of inositol 1,3,4,5-tetrakisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands may both result indirectly from receptor-stimulated release of inositol 1,4,5-trisphosphate from phosphatidylinositol 4,5-bisphosphate. Biochem J. 1986 Sep 1;238(2):507–516. doi: 10.1042/bj2380507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P. J., Drummond A. H. Formation of inositol phosphate isomers in GH3 pituitary tumour cells stimulated with thyrotropin-releasing hormone. Acute effects of lithium ions. Biochem J. 1987 Dec 1;248(2):463–470. doi: 10.1042/bj2480463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilundain A., O'Brien J. A., Burton K. A., Sepúlveda F. V. Inositol trisphosphate and calcium mobilisation in permeabilised enterocytes. Biochim Biophys Acta. 1987 Jan 9;896(1):113–116. doi: 10.1016/0005-2736(87)90363-4. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Berridge M. J. Specificity of inositol phosphate-stimulated Ca2+ mobilization from Swiss-mouse 3T3 cells. Biochem J. 1986 Nov 15;240(1):301–304. doi: 10.1042/bj2400301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Heslop J. P., Berridge M. J. Inositol(3,4)bisphosphate and inositol(1,3)bisphosphate in GH4 cells--evidence for complex breakdown of inositol(1,3,4)trisphosphate. Biochem Biophys Res Commun. 1987 Feb 27;143(1):353–359. doi: 10.1016/0006-291x(87)90672-3. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellett G. L., Jamal A., Robertson J. P., Wollen N. The acute regulation of glucose absorption, transport and metabolism in rat small intestine by insulin in vivo. Biochem J. 1984 May 1;219(3):1027–1035. doi: 10.1042/bj2191027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. E., Stephens L. R., Hawkins P. T., Guy G. R., Michell R. H. Multiple metabolic pools of phosphoinositides and phosphatidate in human erythrocytes incubated in a medium that permits rapid transmembrane exchange of phosphate. Biochem J. 1987 May 15;244(1):209–217. doi: 10.1042/bj2440209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. J., Veum T. L. Adaptive increase in phytate digestibility by phosphorus-deprived rats and the relationship of intestinal phytase (EC 3.1.3.8) and alkaline phosphatase (EC 3.1.3.1) to phytate utilization. Br J Nutr. 1983 Jan;49(1):145–152. doi: 10.1079/bjn19830019. [DOI] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Phillippy B. Q., White K. D., Johnston M. R., Tao S. H., Fox M. R. Preparation of inositol phosphates from sodium phytate by enzymatic and nonenzymatic hydrolysis. Anal Biochem. 1987 Apr;162(1):115–121. doi: 10.1016/0003-2697(87)90015-7. [DOI] [PubMed] [Google Scholar]
- Rana R. S., Sekar M. C., Hokin L. E., MacDonald M. J. A possible role for glucose metabolites in the regulation of inositol-1,4,5-trisphosphate 5-phosphomonoesterase activity in pancreatic islets. J Biol Chem. 1986 Apr 25;261(12):5237–5240. [PubMed] [Google Scholar]
- Rao R. K., Ramakrishnan C. V. Studies on inositolphosphatase in rat small intestine. Enzyme. 1985;33(4):205–215. doi: 10.1159/000469435. [DOI] [PubMed] [Google Scholar]
- Sharps E. S., McCarl R. L. A high-performance liquid chromatographic method to measure 32P incorporation into phosphorylated metabolites in cultured cells. Anal Biochem. 1982 Aug;124(2):421–424. doi: 10.1016/0003-2697(82)90059-8. [DOI] [PubMed] [Google Scholar]
- Shears S. B., Kirk C. J., Michell R. H. The pathway of myo-inositol 1,3,4-trisphosphate dephosphorylation in liver. Biochem J. 1987 Dec 15;248(3):977–980. doi: 10.1042/bj2480977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears S. B., Parry J. B., Tang E. K., Irvine R. F., Michell R. H., Kirk C. J. Metabolism of D-myo-inositol 1,3,4,5-tetrakisphosphate by rat liver, including the synthesis of a novel isomer of myo-inositol tetrakisphosphate. Biochem J. 1987 Aug 15;246(1):139–147. doi: 10.1042/bj2460139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears S. B., Storey D. J., Morris A. J., Cubitt A. B., Parry J. B., Michell R. H., Kirk C. J. Dephosphorylation of myo-inositol 1,4,5-trisphosphate and myo-inositol 1,3,4-triphosphate. Biochem J. 1987 Mar 1;242(2):393–402. doi: 10.1042/bj2420393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo M., Jackson T., Lightman S., Hanley M. R. Occurrence and extracellular actions of inositol pentakis- and hexakisphosphate in mammalian brain. Nature. 1987 Dec 17;330(6149):656–658. doi: 10.1038/330656a0. [DOI] [PubMed] [Google Scholar]
- Velasco G., Domínguez P., Shears S. B., Lazo P. S. Permeability properties of isolated enterocytes from rat small intestine. Biochim Biophys Acta. 1986 Dec 19;889(3):361–365. doi: 10.1016/0167-4889(86)90199-0. [DOI] [PubMed] [Google Scholar]
- Velasco G., Iglesias C. F., Domínguez P., Barros F., Gascón S., Lazo P. S. Protein kinase C from small intestine epithelial cells. Biochem Biophys Res Commun. 1986 Sep 30;139(3):875–882. doi: 10.1016/s0006-291x(86)80259-5. [DOI] [PubMed] [Google Scholar]
- Velasco G., Shears S. B., Michell R. H., Lazo P. S. Calcium uptake by intracellular compartments in permeabilised enterocytes. Effect of inositol 1,4,5 trisphosphate. Biochem Biophys Res Commun. 1986 Sep 14;139(2):612–618. doi: 10.1016/s0006-291x(86)80034-1. [DOI] [PubMed] [Google Scholar]
- Wreggett K. A., Howe L. R., Moore J. P., Irvine R. F. Extraction and recovery of inositol phosphates from tissues. Biochem J. 1987 Aug 1;245(3):933–934. doi: 10.1042/bj2450933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Corven E. J., Verbost P. M., de Jong M. D., van Os C. H. Kinetics of ATP-dependent Ca2+ uptake by permeabilized rat enterocytes. Effects of inositol 1,4,5-trisphosphate. Cell Calcium. 1987 Jun;8(3):197–206. doi: 10.1016/0143-4160(87)90018-2. [DOI] [PubMed] [Google Scholar]


