Abstract
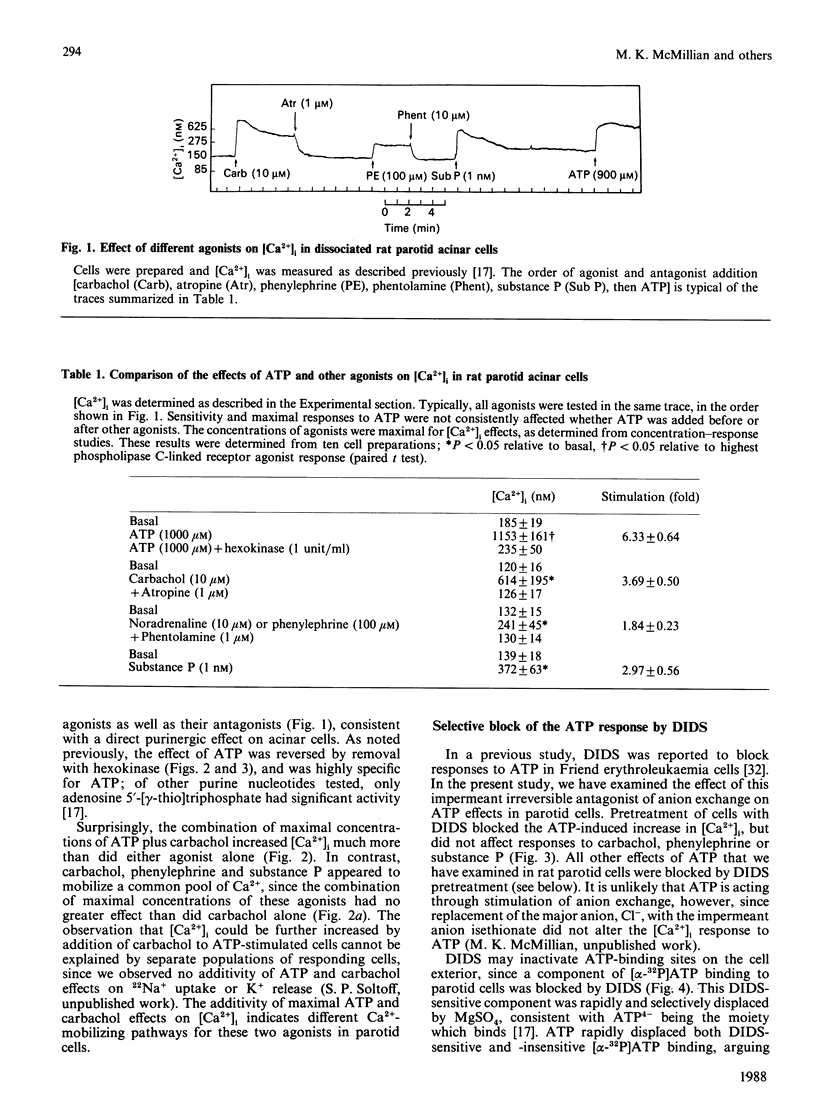
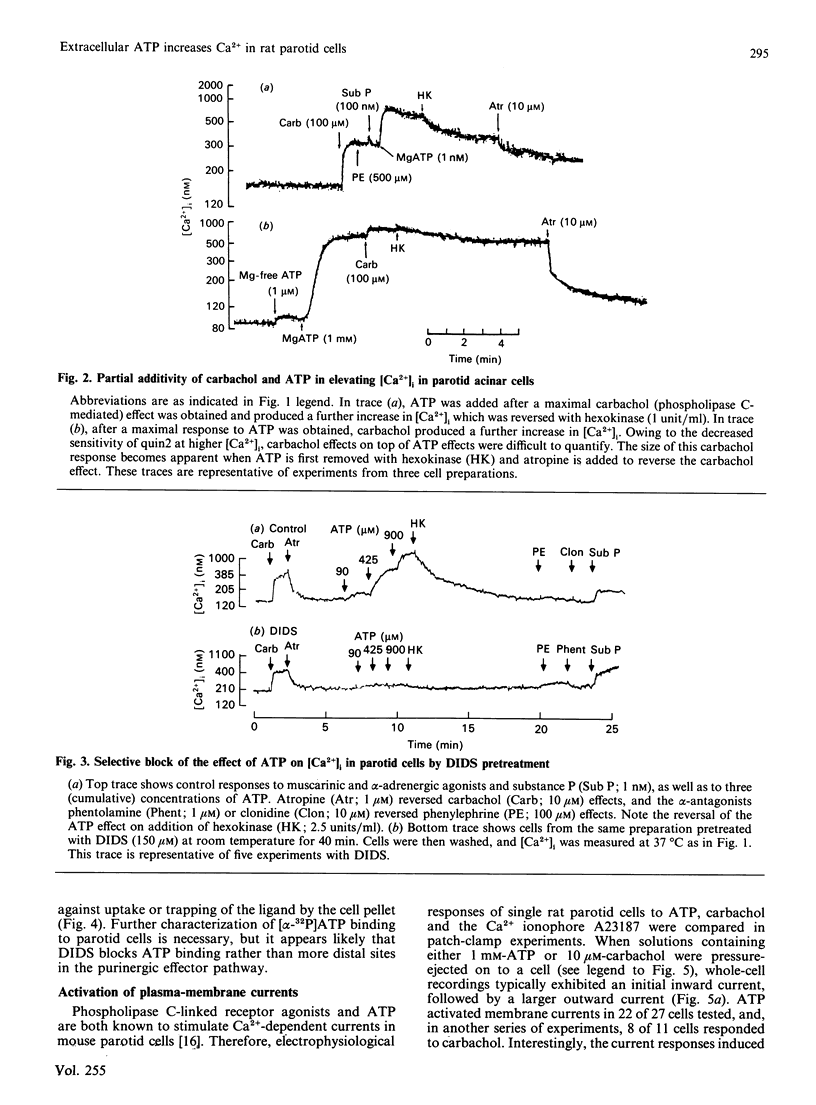
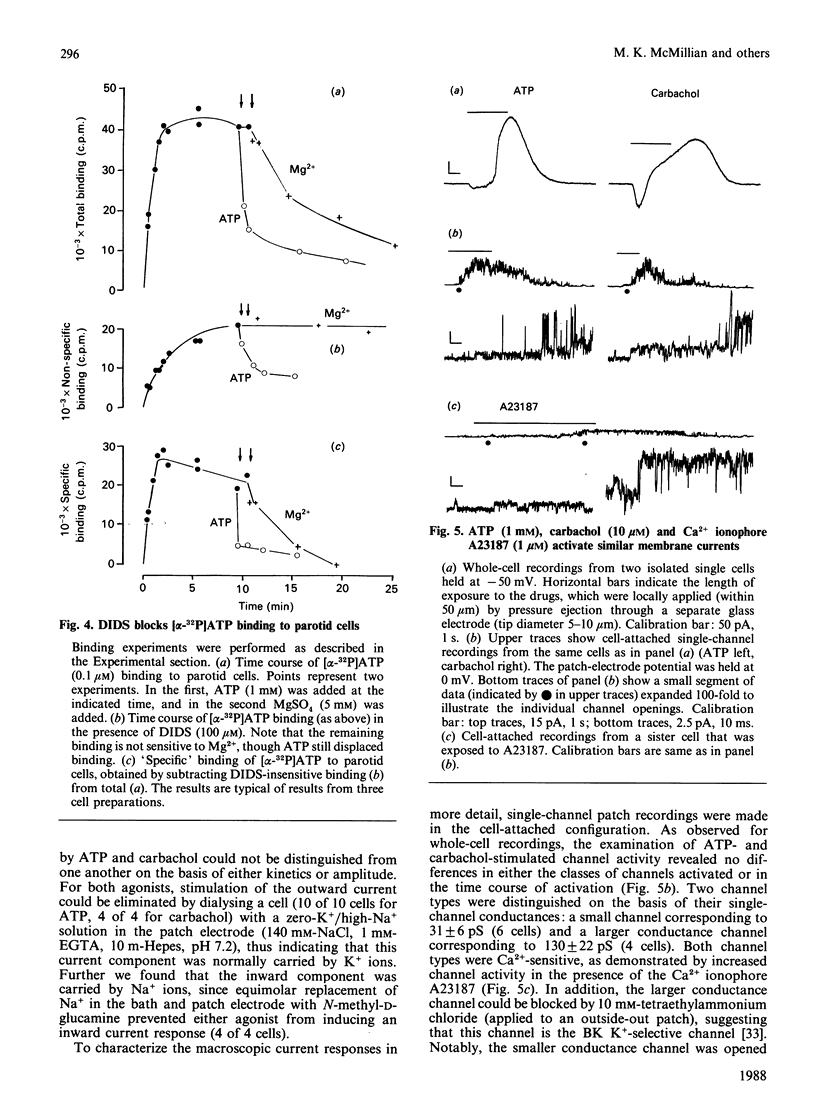
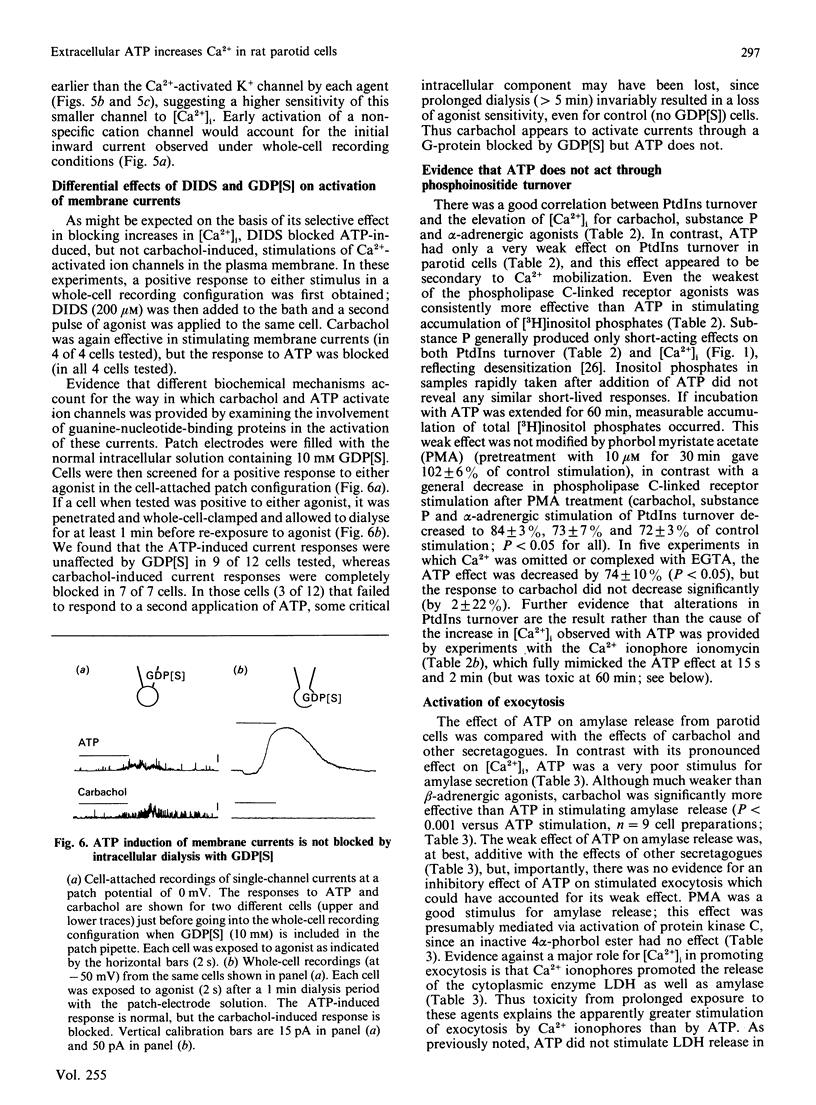
The effects of extracellular ATP on intracellular free calcium concentration [( Ca2+]i), phosphatidylinositol (PtdIns) turnover, amylase release and Ca2+-activated membrane currents were examined in isolated rat parotid acinar cells and contrasted with the effects of receptor agonists known to activate phospholipase C. ATP was more effective than muscarinic and alpha-adrenergic agonists and substance P as a stimulus for elevating [Ca2+]i (as measured with quin2). The ATP effect was selectively antagonized by pretreating parotid cells with the impermeant anion-exchange blocker 4,4'-di-isothiocyano-2,2'-stilbenedisulphonate (DIDS), which also inhibited binding of [alpha-32P]ATP to parotid cells. By elevating [Ca2+]i, ATP and the muscarinic agonist carbachol both activated Ca2+-sensitive membrane currents, which were measured by whole-cell and cell-attached patch-clamp recordings. However, there were marked contrasts between the effects of ATP and the receptor agonists linked to phospholipase C, as follows. (1) Although the combination of maximally effective concentrations of carbachol, substance P and phenylephrine had no greater effect on [Ca2+]i than did carbachol alone, there was some additivity between maximal ATP and carbachol effects. (2) Intracellular dialysis with guanosine 5'-[beta-thio]diphosphate did not block activation of ion channels by ATP, but did block channel activation by the muscarinic agonist carbachol. This suggests that a G-protein is involved in the muscarinic response, but not in the response to ATP. (3) Despite its pronounced effect on [Ca2+]i, ATP had little effect on PtdIns turnover in these cells, in contrast with the effects of carbachol and other Ca2+-mobilizing agents. (4) Although ATP was able to stimulate amylase release from parotid acinar cells, the stimulation was only 33 +/- 9% of that obtained with phospholipase C-linked receptor agonists. These differences suggest that ATP increases [Ca2+]i through specific activation of a pathway which is distinct from that shared by the classical phospholipase C-linked receptor agonists.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. L., Hanley M. R. The effects of substance P and related peptides on alpha-amylase release from rat parotid gland slices. Br J Pharmacol. 1981 Jun;73(2):517–523. doi: 10.1111/j.1476-5381.1981.tb10451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of quinine and apamin on the calcium-dependent potassium permeability of mammalian hepatocytes and red cells. J Physiol. 1981 Aug;317:67–90. doi: 10.1113/jphysiol.1981.sp013814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher F. R. Calcium and cyclic nucleotides in the regulation of secretion from the rat parotid by autonomic agonists. Adv Cyclic Nucleotide Res. 1978;9:707–721. [PubMed] [Google Scholar]
- Butcher F. R., Putney J. W., Jr Regulation of parotid gland function by cyclic nucleotides and calcium. Adv Cyclic Nucleotide Res. 1980;13:215–249. [PubMed] [Google Scholar]
- Chahwala S. B., Cantley L. C. Extracellular ATP induces ion fluxes and inhibits growth of Friend erythroleukemia cells. J Biol Chem. 1984 Nov 25;259(22):13717–13722. [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Cobbold P., Woods N., Wainwright J., Cuthbertson R. Single cell measurements in research on calcium-mobilising purinoceptors. J Recept Res. 1988;8(1-4):481–491. doi: 10.3109/10799898809049006. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. The ATP4- receptor of rat mast cells. Biochem J. 1980 Jun 15;188(3):789–798. doi: 10.1042/bj1880789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak G. R. Extracellular ATP activates polyphosphoinositide breakdown and Ca2+ mobilization in Ehrlich ascites tumor cells. Arch Biochem Biophys. 1986 Feb 15;245(1):84–95. doi: 10.1016/0003-9861(86)90192-x. [DOI] [PubMed] [Google Scholar]
- Fowler C. J., Court J. A., Tiger G., Björklund P. E., Candy J. M. Stimulation of inositol phospholipid breakdown in rat cortical and hippocampal miniprisms by noradrenaline, 5-hydroxytryptamine and carbachol: some methodological aspects. Pharmacol Toxicol. 1987 Apr;60(4):274–279. doi: 10.1111/j.1600-0773.1987.tb01751.x. [DOI] [PubMed] [Google Scholar]
- Friel D. D., Bean B. P. Two ATP-activated conductances in bullfrog atrial cells. J Gen Physiol. 1988 Jan;91(1):1–27. doi: 10.1085/jgp.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher D. V. Are there purinergic receptors on parotid acinar cells? Nature. 1982 Mar 4;296(5852):83–86. doi: 10.1038/296083a0. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston D. A., Burnstock G., Vanhoutte P. M. Different P2-purinergic receptor subtypes of endothelium and smooth muscle in canine blood vessels. J Pharmacol Exp Ther. 1987 May;241(2):501–506. [PubMed] [Google Scholar]
- Irving H. R., Exton J. H. Phosphatidylcholine breakdown in rat liver plasma membranes. Roles of guanine nucleotides and P2-purinergic agonists. J Biol Chem. 1987 Mar 15;262(8):3440–3443. [PubMed] [Google Scholar]
- Iwatsuki N., Maruyama Y., Matsumoto O., Nishiyama A. Activation of Ca2+-dependent Cl- and K+ conductances in rat and mouse parotid acinar cells. Jpn J Physiol. 1985;35(6):933–944. doi: 10.2170/jjphysiol.35.933. [DOI] [PubMed] [Google Scholar]
- Kanagasuntheram P., Randle P. J. Calcium metabolism and amylase release in rat parotid acinar cells. Biochem J. 1976 Dec 15;160(3):547–564. doi: 10.1042/bj1600547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., De Wulf H. Characterization of the liver P2-purinoceptor involved in the activation of glycogen phosphorylase. Biochem J. 1986 Dec 1;240(2):367–371. doi: 10.1042/bj2400367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa S., Endo J., Kubo R., Kametani F. Inhibitory effects of 4,4'-diisothiocyanostilbene-2,2'-disulfonate (DIDS) on the ADP-stimulated aggregation of gel-filtered bovine blood platelets. Biochem Biophys Res Commun. 1983 Feb 28;111(1):306–311. doi: 10.1016/s0006-291x(83)80152-1. [DOI] [PubMed] [Google Scholar]
- Krell H., Ermisch N., Kasperek S., Pfaff E. On the mechanisms of ATP-induced and succinate-induced redistribution of cations in isolated rat liver cells. Eur J Biochem. 1983 Mar 15;131(2):247–254. doi: 10.1111/j.1432-1033.1983.tb07256.x. [DOI] [PubMed] [Google Scholar]
- McMillian M. K., Soltoff S. P., Cantley L. C., Talamo B. R. Extracellular ATP elevates intracellular free calcium in rat parotid acinar cells. Biochem Biophys Res Commun. 1987 Dec 16;149(2):523–530. doi: 10.1016/0006-291x(87)90399-8. [DOI] [PubMed] [Google Scholar]
- McMillian M. K., Soltoff S. P., Talamo B. R. Rapid desensitization of substance P- but not carbachol-induced increases in inositol trisphosphate and intracellular Ca++ in rat parotid acinar cells. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1017–1024. doi: 10.1016/s0006-291x(87)80233-4. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Rink T. J. The effects of substance P and carbachol on inositol tris- and tetrakisphosphate formation and cytosolic free calcium in rat parotid acinar cells. A correlation between inositol phosphate levels and calcium entry. J Biol Chem. 1987 Nov 5;262(31):14912–14916. [PubMed] [Google Scholar]
- Nauntofte B., Poulsen J. H. Effects of Ca2+ and furosemide on Cl- transport and O2 uptake in rat parotid acini. Am J Physiol. 1986 Aug;251(2 Pt 1):C175–C185. doi: 10.1152/ajpcell.1986.251.2.C175. [DOI] [PubMed] [Google Scholar]
- Nicchitta C. V., Joseph S. K., Williamson J. R. GTP-mediated Ca2+ release in rough endoplasmic reticulum. Correlation with a GTP-sensitive increase in membrane permeability. Biochem J. 1987 Dec 15;248(3):741–747. doi: 10.1042/bj2480741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Okajima F., Tokumitsu Y., Kondo Y., Ui M. P2-purinergic receptors are coupled to two signal transduction systems leading to inhibition of cAMP generation and to production of inositol trisphosphate in rat hepatocytes. J Biol Chem. 1987 Oct 5;262(28):13483–13490. [PubMed] [Google Scholar]
- Petersen O. H., Maruyama Y. Calcium-activated potassium channels and their role in secretion. Nature. 1984 Feb 23;307(5953):693–696. doi: 10.1038/307693a0. [DOI] [PubMed] [Google Scholar]
- Richards N. W., Allbee W. E., Gaginella T. S., Wallace L. J. Exogenous ATP-stimulated calcium uptake in isolated rat intestinal epithelial cells. Life Sci. 1987 Apr 27;40(17):1665–1672. doi: 10.1016/0024-3205(87)90015-4. [DOI] [PubMed] [Google Scholar]
- Richardson P. J., Brown S. J. ATP release from affinity-purified rat cholinergic nerve terminals. J Neurochem. 1987 Feb;48(2):622–630. doi: 10.1111/j.1471-4159.1987.tb04138.x. [DOI] [PubMed] [Google Scholar]
- Sage S. O., Rink T. J. The kinetics of changes in intracellular calcium concentration in fura-2-loaded human platelets. J Biol Chem. 1987 Dec 5;262(34):16364–16369. [PubMed] [Google Scholar]
- Staddon J. M., McGivan J. D. Effects of ATP and adenosine addition on activity of oxoglutarate dehydrogenase and the concentration of cytoplasmic free Ca2+ in rat hepatocytes. Eur J Biochem. 1985 Sep 16;151(3):567–572. doi: 10.1111/j.1432-1033.1985.tb09141.x. [DOI] [PubMed] [Google Scholar]
- Steinberg T. H., Silverstein S. C. Extracellular ATP4- promotes cation fluxes in the J774 mouse macrophage cell line. J Biol Chem. 1987 Mar 5;262(7):3118–3122. [PubMed] [Google Scholar]
- Sugiya H., Tennes K. A., Putney J. W., Jr Homologous desensitization of substance-P-induced inositol polyphosphate formation in rat parotid acinar cells. Biochem J. 1987 Jun 15;244(3):647–653. doi: 10.1042/bj2440647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma T., Ichida T. Does cyclic AMP mobilize Ca2+ for amylase secretion from rat parotid cells? Biochim Biophys Acta. 1986 Jun 16;887(1):113–117. doi: 10.1016/0167-4889(86)90130-8. [DOI] [PubMed] [Google Scholar]
- Taylor C. W., Merritt J. E., Putney J. W., Jr, Rubin R. P. A guanine nucleotide-dependent regulatory protein couples substance P receptors to phospholipase C in rat parotid gland. Biochem Biophys Res Commun. 1986 Apr 14;136(1):362–368. doi: 10.1016/0006-291x(86)90919-8. [DOI] [PubMed] [Google Scholar]
- Thor H., Hartzell P., Orrenius S. Potentiation of oxidative cell injury in hepatocytes which have accumulated Ca2+. J Biol Chem. 1984 May 25;259(10):6612–6615. [PubMed] [Google Scholar]
- Weisman G. A., De B. K., Friedberg I., Pritchard R. S., Heppel L. A. Cellular responses to external ATP which precede an increase in nucleotide permeability in transformed cells. J Cell Physiol. 1984 May;119(2):211–219. doi: 10.1002/jcp.1041190211. [DOI] [PubMed] [Google Scholar]
- White T. D. Characteristics of neuronal release of ATP. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8(4-6):487–493. doi: 10.1016/0278-5846(84)90005-8. [DOI] [PubMed] [Google Scholar]


