Abstract
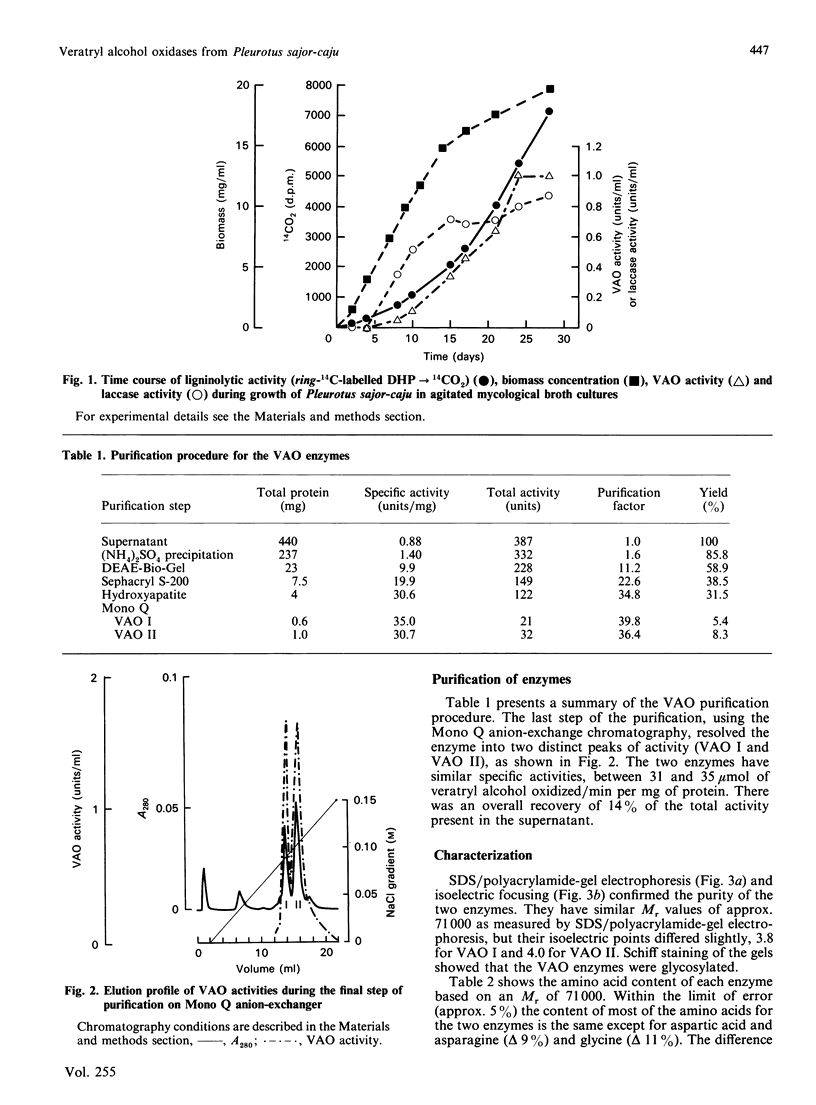
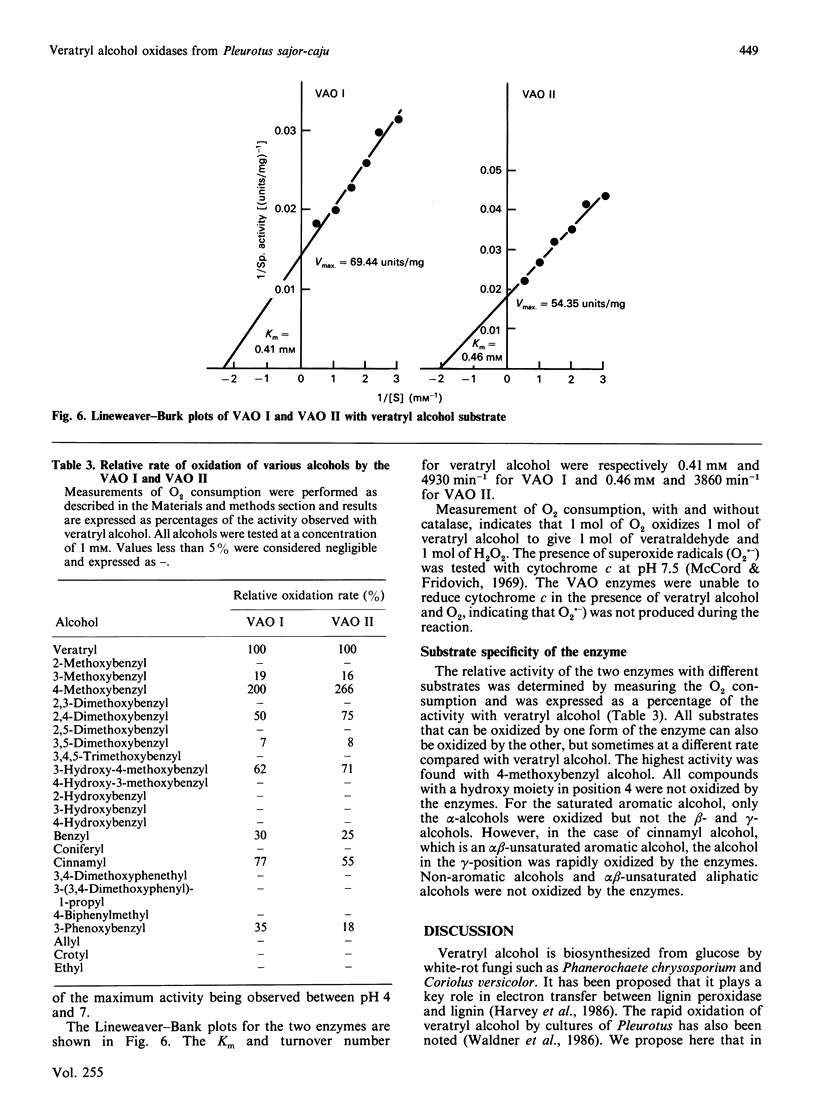
The basidiomycete Pleurotus sajor-caju mineralizes ring-14C-labelled lignin (dehydrogenative polymer) when grown in mycological broth. Under these conditions, two veratryl alcohol oxidase (VAO) enzymes were found in the culture medium. They oxidized a number of aromatic alcohols to aldehydes and reduced O2 to H2O2. The enzymes were purified by ion-exchange and gel-permeation chromatography. The final step of purification on Mono Q resolved the activity into two peaks (VAO I and VAO II). Both enzymes had the same Mr, approx. 71,000, but their isoelectric points differed slightly, 3.8 for VAO I and 4.0 for VAO II. Their amino acid compositions were similar except for aspartic acid/asparagine and glycine. Both enzymes are glycoproteins and contain flavin prosthetic groups. Their pH optima were around 5, and kinetic constants and specificities were similar. 4-Methoxybenzyl alcohol was oxidized the most rapidly, followed by veratryl alcohol. Not all aromatic alcohols were oxidized, neither were non-aromatic alcohols. Cinnamyl alcohol was oxidized at the gamma position. The VAO enzymes thus represent a significantly different route for veratryl alcohol oxidation from that catalysed by the previously found lignin peroxidases from Phanerochaete chrysosporium. The role of the oxidases in biodegradation might be to produce H2O2 during oxidation of lignin fragments.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- FARMER V. C., HENDERSON M. E., RUSSELL J. D. Aromatic-alcohol-oxidase activity in the growth medium of Polystictus versicolor. Biochem J. 1960 Feb;74:257–262. doi: 10.1042/bj0740257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K. Factors Involved in the Regulation of a Ligninase Activity in Phanerochaete chrysosporium. Appl Environ Microbiol. 1985 Feb;49(2):299–304. doi: 10.1128/aem.49.2.299-304.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K., Farrell R. L. Role of Veratryl Alcohol in Regulating Ligninase Activity in Phanerochaete chrysosporium. Appl Environ Microbiol. 1986 Aug;52(2):251–254. doi: 10.1128/aem.52.2.251-254.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J. K., Morgan M. A., Mayfield M. B., Kuwahara M., Gold M. H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J Biol Chem. 1971 Oct 25;246(20):6339–6346. [PubMed] [Google Scholar]
- Kersten P. J., Kirk T. K. Involvement of a new enzyme, glyoxal oxidase, in extracellular H2O2 production by Phanerochaete chrysosporium. J Bacteriol. 1987 May;169(5):2195–2201. doi: 10.1128/jb.169.5.2195-2201.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Connors W. J., Bleam R. D., Hackett W. F., Zeikus J. G. Preparation and microbial decomposition of synthetic [14C]ligins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2515–2519. doi: 10.1073/pnas.72.7.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]



