Abstract
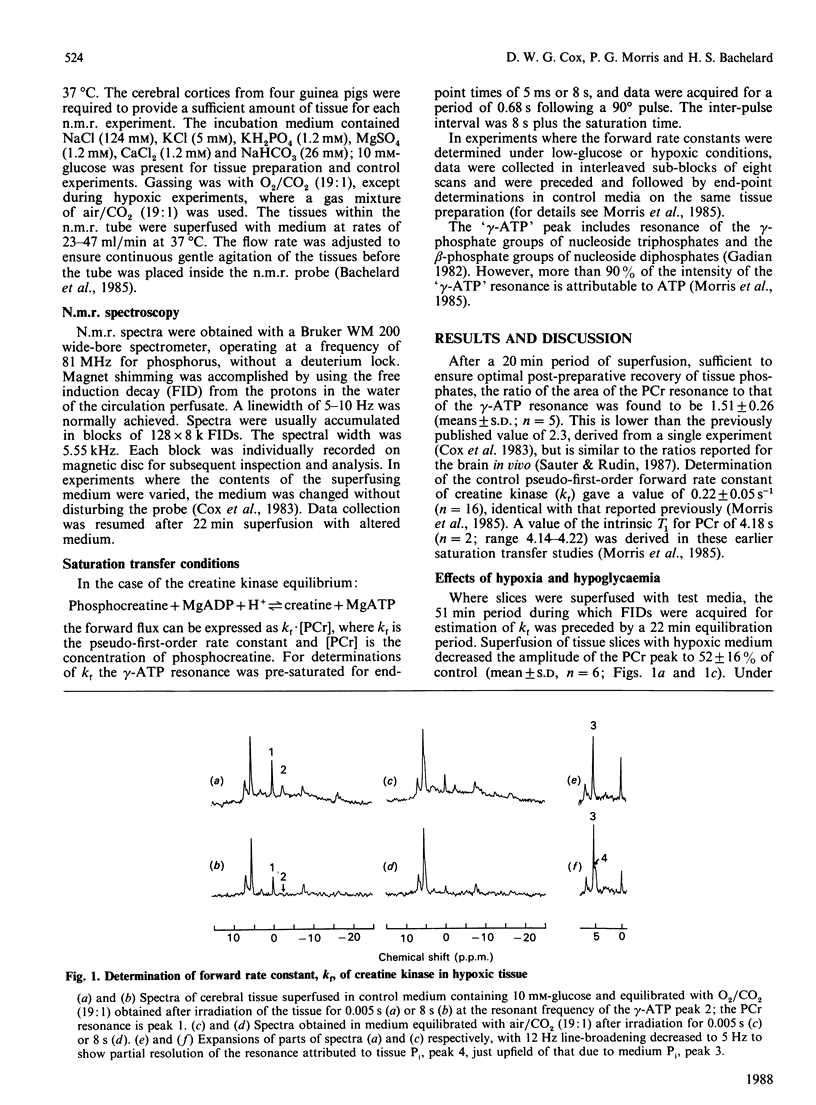
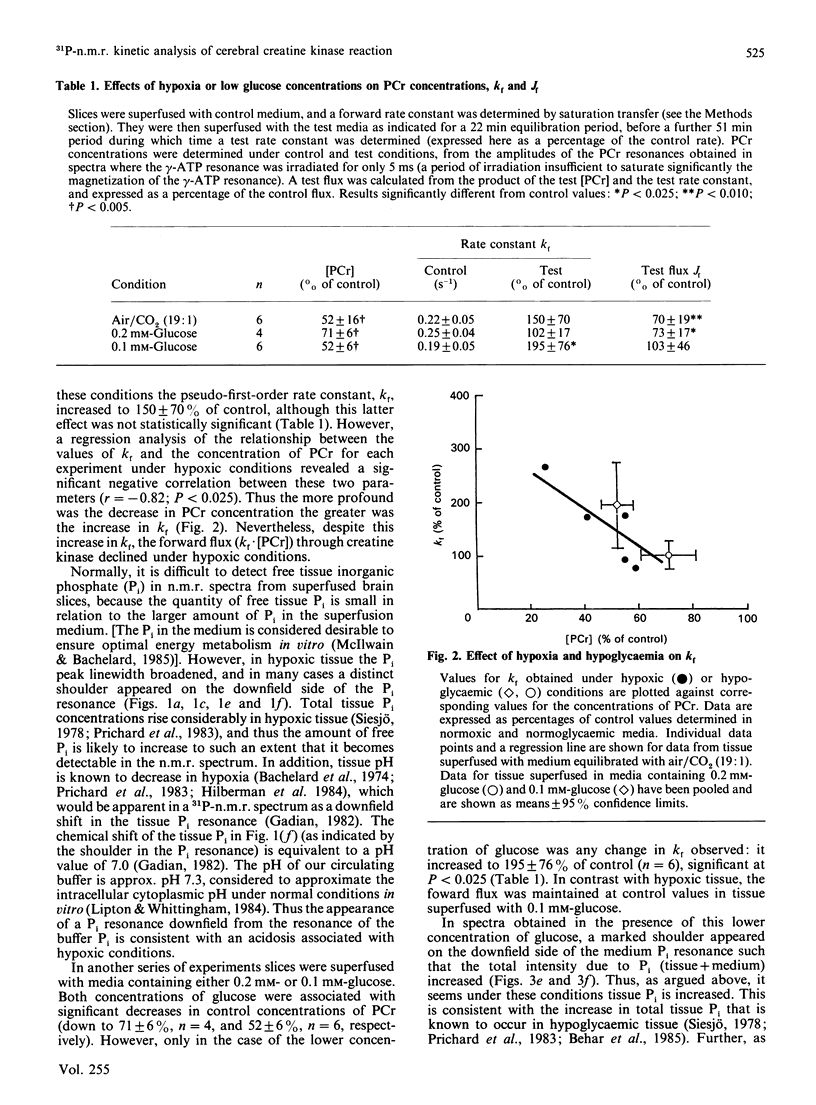
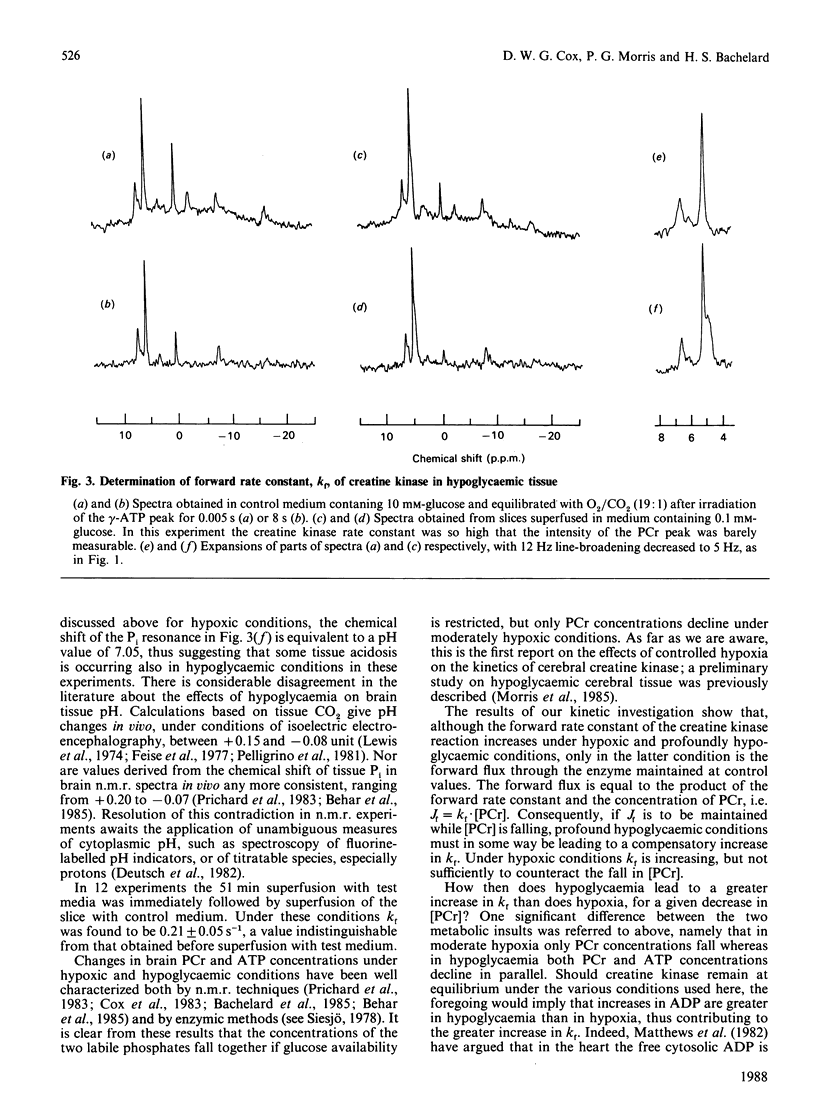
1. The tissue concentration of phosphocreatine (PCr) and the pseudo-first-order rate constant of creatine kinase (kf) were monitored in superfused guinea-pig brain slices in vitro by using 31P-n.m.r. techniques. 2. Superfusion of slices in low oxygen partial pressure (pO2 approx. 16 kPa) decreased tissue PCr concentrations by 48% but ATP concentrations were unchanged. Regression analysis revealed a significant negative correlation between the PCr concentration in hypoxic tissue and the increase in the rate constant, kf. Nevertheless the forward flux through the enzyme (Jf = kf.[PCr]) declined under these conditions. 3. Lowering the glucose concentration to 0.2 or 0.1 mM decreased PCr concentrations by 29% and 48% respectively; here ATP concentrations as well as PCr concentrations also decreased. Only in the presence of the lower glucose concentration (0.1 mM) was kf increased. However, unlike the situation in hypoxic tissue, Jf was maintained at control rates. 4. In spectra obtained in the presence of low oxygen or low glucose concentrations, a resonance attributable to tissue inorganic phosphate became dectectable. This observation is discussed in terms of known changes in tissue phosphate concentrations and possible alterations in cytoplasmic pH.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachelard H. S., Cox D. W., Feeney J., Morris P. G. 31P nuclear-magnetic-resonance studies on superfused cerebral tissues. Biochem Soc Trans. 1985 Oct;13(5):835–839. doi: 10.1042/bst0130835. [DOI] [PubMed] [Google Scholar]
- Bachelard H. S., Lewis L. D., Pontén U., Siesjö B. K. Mechanisms activating glycolysis in the brain in arterial hypoxia. J Neurochem. 1974 Mar;22(3):395–401. doi: 10.1111/j.1471-4159.1974.tb07605.x. [DOI] [PubMed] [Google Scholar]
- Behar K. L., den Hollander J. A., Petroff O. A., Hetherington H. P., Prichard J. W., Shulman R. G. Effect of hypoglycemic encephalopathy upon amino acids, high-energy phosphates, and pHi in the rat brain in vivo: detection by sequential 1H and 31P NMR spectroscopy. J Neurochem. 1985 Apr;44(4):1045–1055. doi: 10.1111/j.1471-4159.1985.tb08723.x. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Morris P. G., Feeney J., Bachelard H. S. 31P-n.m.r. studies on cerebral energy metabolism under conditions of hypoglycaemia and hypoxia in vitro. Biochem J. 1983 May 15;212(2):365–370. doi: 10.1042/bj2120365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch C., Taylor J. S., Wilson D. F. Regulation of intracellular pH by human peripheral blood lymphocytes as measured by 19F NMR. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7944–7948. doi: 10.1073/pnas.79.24.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feise G., Kogure K., Busto K. R., Scheinberg P., Reinmuth O. M. Effect of insulin hypoglycemia upon cerebral energy metabolism and EEG activity in the rat. Brain Res. 1977 May 6;126(2):263–280. doi: 10.1016/0006-8993(77)90725-9. [DOI] [PubMed] [Google Scholar]
- Hilberman M., Subramanian V. H., Haselgrove J., Cone J. B., Egan J. W., Gyulai L., Chance B. In vivo time-resolved brain phosphorus nuclear magnetic resonance. J Cereb Blood Flow Metab. 1984 Sep;4(3):334–342. doi: 10.1038/jcbfm.1984.50. [DOI] [PubMed] [Google Scholar]
- Lewis L. D., Ljunggren B., Norberg K., Siesjö B. K. Changes in carbohydrate substrates, amino acids and ammonia in the brain during insulin-induced hypoglycemia. J Neurochem. 1974 Oct;23(4):659–671. doi: 10.1111/j.1471-4159.1974.tb04389.x. [DOI] [PubMed] [Google Scholar]
- Matthews P. M., Bland J. L., Gadian D. G., Radda G. K. A 31P-NMR saturation transfer study of the regulation of creatine kinase in the rat heart. Biochim Biophys Acta. 1982 Nov 17;721(3):312–320. doi: 10.1016/0167-4889(82)90084-2. [DOI] [PubMed] [Google Scholar]
- Morris P. G., Feeney J., Cox D. W., Bachelard H. S. 31P-saturation-transfer nuclear-magnetic-resonance measurements of phosphocreatine turnover in guinea-pig brain slices. Biochem J. 1985 May 1;227(3):777–782. doi: 10.1042/bj2270777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelligrino D., Almquist L. O., Siesjö B. K. Effects of insulin-induced hypoglycemia on intracellular pH and impedance in the cerebral cortex of the rat. Brain Res. 1981 Sep 21;221(1):129–147. doi: 10.1016/0006-8993(81)91068-4. [DOI] [PubMed] [Google Scholar]
- Prichard J. W., Alger J. R., Behar K. L., Petroff O. A., Shulman R. G. Cerebral metabolic studies in vivo by 31P NMR. Proc Natl Acad Sci U S A. 1983 May;80(9):2748–2751. doi: 10.1073/pnas.80.9.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter A., Rudin M. Effects of calcium antagonists on high-energy phosphates in ischemic rat brain measured by 31P NMR spectroscopy. Magn Reson Med. 1987 Jan;4(1):1–8. doi: 10.1002/mrm.1910040102. [DOI] [PubMed] [Google Scholar]


