Abstract
Mass extinctions are major influences on both the phylogenetic structure of the modern biota and our ability to reconstruct broad-based patterns of evolutionary history. The most recent mass extinction is also the most famous—that which implicates a bolide impact in defining the Cretaceous/Palaeogene boundary (K/Pg). Although the biotic effects of this event receive intensive scrutiny, certain ecologically important and diverse groups remain woefully understudied. One such group is the freshwater ray-finned fishes (Actinopterygii). These fish represent 25% of modern vertebrate diversity, yet the isolated and fragmentary nature of their K/Pg fossil record limits our understanding of their diversity dynamics across this event. Here, we address this problem using diversification analysis of molecular-based phylogenies alongside a morphotype analysis of fossils recovered from a unique site in the Denver Basin of western North America that provides unprecedented K/Pg resolution. Our results reveal previously unrecognized signals of post-K/Pg diversification in freshwater clades and suggest that the change was driven by localized and sporadic patterns of extinction. Supported inferences regarding the effects of the K/Pg event on freshwater fish also inform our expectations of how freshwater faunas might recover from the current biodiversity crisis.
Keywords: extinction, freshwater, Actinopterygii, fish, fossil, diversification
1. Introduction
Extinction as a scientific pursuit may be traced to Georges Cuvier and his late eighteenth-century assertion that the mastodon fossils of the Paris Basin proved the failure of the modern biota to represent Creation in its totality [1,2]. Today, extinction studies flourish and bear a practical importance given the increasingly robust hypothesis that we have entered a sixth mass extinction [3–5]. The empirical basis of these studies draws heavily from the fossil record, comparative genomics or some combination of the two (for recent examples for the K/Pg, see [6,7]). Despite challenges posed by the sensitivity of such analyses to time priors [8], the ‘best practice’ for integrating molecular and morphological evidence is a tip-calibrated Bayesian analysis with dense sampling of both extant and fossil taxa. Few K/Pg studies approach this analytical ideal, but those that do use a tip-calibration framework with extant and fossil specimens scored into morphological matrices (with or without a molecular scaffold; e.g. [9]).
The quality of tip-calibrations depends, at least in part, on access to well-preserved fossils [8]. Here, quality refers not only to skeletal completeness (scoring a large percentage of analysed characters to improve phylogenetic stability) but also to a temporally and phylogenetically diverse sample. Such diversity is critical, especially when evolutionary rates vary [8,10]. The problem is that many vertebrate groups lack such a fossil record. This is certainly the case for the K/Pg record of freshwater actinopterygians. Despite a few notable exceptions (e.g. [11–14]), this record is dominated by isolated and fragmentary elements that accumulated from high-energy fluvial environments and were collected via screen-washing techniques [15]. Some such elements preserve established synapomorphies, thus permitting their integration within existing phylogenies (e.g. [16]). Most established skeletal apomorphies for freshwater actinopterygians, however, inform only highly inclusive areas of the tree, constraining their ability to test taxonomically refined hypotheses of extinction, survivorship and diversification. Until more discriminating patterns of skeletal variation can be established for these groups—a Herculean task considering diversity levels—we either must be content with excluding this dense fossil record from important and ongoing discussions of extinction dynamics or we must consider this resource from novel perspectives.
Here, we pursue the latter option with a study that combines parataxonomic morphotype analysis of isolated fossils of freshwater actinopterygians with comparative genomics, using Bayesian methods and molecular phylogenies to search for patterns of diversification correlated with the K/Pg mass extinction. More specifically, we use this heterogenous approach to test the existing hypothesis that freshwater fish lineages, unlike the better preserved (and more extensively studied) marine forms (e.g. [6,17–19]), were not significantly affected by the K/Pg mass extinction event [20–22]. This effort represents an advance over previous morphotype analyses in that the fossils examined here are from a unique study area in the Denver Basin (Colorado, USA) that preserves freshwater actinopterygian microfossils in two closely associated sites (<400 m apart geographically) that are preserved in a similar facies and stratigraphically bracket the K/Pg boundary, with a temporal separation of only approximately 185 thousand years (kyr) [23–25]. This proximity allows unprecedented control for assessing the K/Pg influence on freshwater fish communities. The isolated nature of the specimens constituting the hyper-localized fossil data from this unique locality cannot yet be fully incorporated into a phylogenetic framework, which also means that they cannot yet be fully integrated with our global phylogenomic analysis. Still, the synthesis of the two patterns can provide reciprocal illumination that fertilizes the growth of novel insights, perspectives and hypotheses. Results indicate that although the K/Pg did not initiate the dramatic turnover in freshwater fish diversity that it did for terrestrial vertebrate groups, there were detectable effects. Molecular phylogenies of some now-dominant freshwater clades indicate the extinction opened ecological opportunities that induced subsequent adaptive radiation. Furthermore, temporally and geographically constrained fossil analysis suggests that this clade-specific diversification was driven by local changes in community diversity.
2. Methods
(a). Diversification rate analysis
Mass extinctions produce high rates of turnover in the taxonomic composition of global diversity [26]. This turnover is driven in part by the extinction-led opening of ecological opportunities followed by opportunistic diversification [27] (but see [28]). Given inherent analytical advantages, molecular phylogenies with fossil calibration are often the preferred choice for testing whether diversification rates increase or decrease around a given extinction event. The published time-calibrated phylogeny of Rabosky et al. [29] (hereafter R18) covers the breadth of the actinopterygian crown and includes over 11 000 tip taxa. Although it does not focus exclusively on freshwater clades, the phylogeny is one of the most inclusive samples of freshwater taxa available. We extracted subtrees from the time-calibrated tree of R18 and analysed diversification rate-through-time of several actinopterygian clades that predominantly inhabit fresh water to test for changes in diversification rate after the K/Pg mass extinction event in those taxa.
To calculate diversification rate-through-time, we used Bayesian analysis of macroevolutionary mixtures (BAMM v. 2.5.0 [30]). We chose BAMM over other analytical tools because it can incorporate clade-specific information on sampling in phylogenetic datasets, a potentially important influence on reconstructing patterns of diversification [31,32]. We downloaded the 11 638-tip time-calibrated actinopterygian tree of R18 and extracted subtrees for major freshwater clades (including Cypriniformes, Characiformes, Siluriformes, Osteoglossomorpha, Esociformes/Salmoniformes and Gymnotiformes) using the R package ape v. 5.7-1 [33]. These clades were chosen for their diversity and the age of their initial divergence. Some focal clades include species that move into brackish or near-shore marine water, and some include fully marine taxa (Siluriformes and Salmoniformes). These marine species were included because the ancestral habitat of each of these focal clades is reconstructed as fresh water [34]. We also included two clades whose inferred ancestral habitat is marine but include extant freshwater taxa (Anabantaria and Ovalentaria [34]). Ovalentaria includes many marine taxa but also two large freshwater clades, Cyprinodontiformes and Cichlidae. All focal trees include a substantial percentage of extant diversity: Cypriniformes (1687 species, 37% of extant diversity), Characiformes (483 species, 22%), Siluriformes (1155 species, 30%), Osteoglossomorpha (99 species, 39%), Esociformes/Salmoniformes (97 species, 40%), Gymnotiformes (43 species, 18%), Anabantaria (157 species, 40%) and Ovalentaria (2276 species, 42%). Subspecies were removed and names were updated primarily according to FishBase [35–37] (see electronic supplementary material for details).
We estimated sampling probabilities for each focal clade after all species names were updated. Sampling probabilities were based on genus-level species counts from FishBase (using rfishbase v. 4.1.2 [35–38]) assembled with protocols detailed in the electronic supplementary material. For Cypriniformes and Ovalentaria, considerable polyphyly in several genera (Raiamas, Rhodeus and Haplochromis) drove sampling fractions that encompassed highly inclusive clades. This polyphyly was caused by single species placed far from other congeneric species: Ra. intermedius and Rh. sinensis in Cypriniformes and H. maculipinna in Ovalentaria. Analyses were run including and excluding these species.
We set priors for focal trees using the R package BAMMtools v. 2.1.10 [39] and ran all analyses with the expected number of shifts set to 1, 10 and 100. Each analysis ran for 100 million generations with ΔT of 0.01 and outputs recorded every 10 000 generations. Post-processing used a 10% burn-in. After analysis, all runs converged with effective sample sizes for both N-shifts and log likelihood over 200 (calculated using R package coda, v. 0.19-4 [40]). We also ran analyses covering Acanthomorpha to look for a previously noted signal of diversification in marine acanthomorphs shortly after the K/Pg [19]. We corrected names, produced sampling probabilities and ran the BAMM analysis for Acanthomorpha following the same procedures outlined above, but only used expected shifts of 10 and 100 due to the large size of the tree (7168 species; 37% of extant diversity).
We used phylorate plots to search for signals of tree-wide diversification increases associated with the K/Pg. We also searched for signals in less-inclusive subclades that would suggest adaptive radiation in a habitat opened by the K/Pg. In the phylorate plots, a rapid increase in diversification after the K/Pg followed by a decrease approaching the present suggests that a clade expanded into open ecological opportunity [27]. To assess apparent shifts in diversification rate, we used mean phylorate and maximum shift credibility plots. When the credible shift set contained a clearly dominant model, the best shift configuration was used, but because of the large number of taxa in many of the trees, this was only meaningful in a few clades. Because the extant tree is the product of both extinction and speciation, net diversification was used for all analyses.
After the analysis of subtrees using the phylogeny from R18, several follow-up analyses were conducted using the dataset of Alfaro et al. [19] (hereafter A18). For these analyses, we calculated sampling probabilities at a more inclusive taxonomic level (family) because the A18 tree is more sparsely sampled than the R18 tree. The setup for BAMM used the parameters outlined above for other analyses.
(b). Morphotype approach
Whereas global changes in speciation and extinction may leave traces in phylogenies of extant taxa, localized effects might only be detectable with fossils. The extraction of refined diversity data from the fossil record requires a thorough understanding of morphological character evolution and the phylogenetic polarity of preserved features [41]. Such understanding is especially challenging with a fossil record dominated by fragmentary and isolated specimens [42,43]. Although this challenge has been met in K/Pg studies for certain groups (e.g. Squamata; [44]), our understanding of variation in the scales, vertebral centra and tooth-bearing elements that largely comprise the K/Pg record of freshwater actinopterygians has simply not reached the maturity needed to conduct phylogenetically rigorous analyses. In lieu of more robust phylogenetic methods, K/Pg studies of freshwater actinopterygians often employ a parataxonomic morphotype approach (e.g. [45–48]). A significant shortcoming of morphotypes is that their defining features have yet to be fully explored in a phylogenetic system, leaving them with an unclear relationship to the phylogenetic lineages they are intended to inform [42]. An unclear relationship, however, does not mean no relationship at all and morphotype approaches have a deep and ongoing history in K/Pg studies, particularly for groups whose fossils lack an abundance of complex characters—such as plant macrofossils and pollen [49,50].
Existing morphotype studies of freshwater fish are limited in heuristic power by the distribution of Late Cretaceous and Palaeocene localities [48]. Historically, this distribution has forced comparisons over large geographic and temporal distances. Observed differences in morphotype diversity between separated sites may result from many macroevolutionary dynamics, and these confounding effects undoubtedly increase with time and spatial distance, making it difficult to isolate any signal of extinction. The ideal application of the morphotype approach is to compare sites separated only by the extinction boundary. If we are interested in the effect of the K/Pg and must rely (at least for now) on a morphotype approach, it is imperative that compared sites are close geographically and separated by minimal time on either side of the event boundary.
The West Bijou Creek area in the Denver Basin preserves a fine-resolution stratigraphic record that is continuous across the K/Pg boundary [23]. The area includes a precise chronostratigraphic framework with palynostratigraphic biozones; well-resolved magnetochron boundaries; uranium-lead dated volcanic ash layers and a K/Pg boundary with shocked minerals, an iridium anomaly and boundary clay [24,51,52]. The area preserves fossil-rich microsites that bracket the K/Pg boundary, including one microsite with abundant dinosaur material. Two sites, DMNH 3648 and DMNH 2560, sit approximately 4 m below and 9 m above the boundary, respectively [25]. The pre-K/Pg site, DMNH 3648, is in a layer estimated to be approximately 57 kyr before the K/Pg boundary; DMNH 2560 post-dates the K/Pg boundary by approximately 128 kyr [24]. The two localities are separated by less than 400 m and there is little to no facies change across the K/Pg boundary. Both localities are preserved in a fine- to medium-grained, mustard yellow sandstone unit interpreted as a meandering river. Both sites are rich in fish material, including scales, tooth-bearing elements and vertebral centra.
Here, we apply a morphotype approach to actinopterygian vertebral centra from West Bijou (n = 103 from DMNH 3648 and n = 236 from DMNH 2560; see electronic supplementary material for details). We focused on centra because they are consistent in general dimensions across taxa and thus their relative abundance is less likely to be affected by taphonomic bias [45,53]. Furthermore, previous studies of actinopterygian vertebral centra provide a guide for the degree and kind of variation expected between morphotypes (e.g. [45–48,54,55]). Analysed centra were recovered through screen washing and thus were generally small in size (<7 mm maximum dimension).
In our initial analysis, we minimized researcher bias based on specimen provenance by anonymizing all specimens (see electronic supplementary material). We then integrated these anonymized results into a ‘retrospective’ analysis that served as the basis for our primary discussion and conclusions. Centra clearly identifiable as the first centrum of the series (i.e. the atlas) or from the caudal region were excluded to reduce the confounding of serial variation along the vertebral column with intertaxonomic variation [45]. In some teleosts, including otophysans and acanthomorphs, the first vertebral centrum exhibits unique morphological features that make them difficult to associate with morphotypes based on more posterior vertebrae [45]. We also excluded centra that were too worn or damaged to be confidently characterized. Some well-preserved centra showed no clear alignment with other specimens and were categorized as ‘unique’.
We calculated four post-analysis diversity indices between sites (following Wilson [56]) using Past4 v. 4.11 [57]. The diversity indices include two measures of heterogeneity: evenness [58] and equitability [59], and two measures of dominance: Simpson’s (1 − D) [60,61], and Berger–Parker [62]. Both heterogeneity indices approach 1 if all taxa in a sample are in equal abundance but evenness varies more at moderate levels of diversity (10−20 taxa [63,64]). Heterogeneity measures are weighted by the taxonomic richness of a given sample, whereas dominance indices focus on the relative abundance of the most abundant taxa. Simpson’s index increases as the community is less dominated by single or a few taxa, whereas the Berger–Parker index is equal to the proportional abundance of the most abundant species. For each diversity index, we used the ‘unbiased’ setting in Past4 and calculated 95% confidence intervals using the centred boot-strap option. We determined the statistical significance of differences in diversity indices using ‘diversity permutation’ in Past4.
Two additional tests were used as separate assessments of differences in diversity between sites. First, we used Hutcheson’s t‐test [65] implemented through the R package ecolTest v. 0.0.1. This test was developed to statistically compare Shannon diversity indices between two samples [65]. Second, we compared the variance of relative abundance data for each of the sites with Levene’s test (using ‘leveneTest’ from R package car v. 3.1-2 [66]). A community with taxa at equal abundance would have less variance than a community with large differences in relative abundance between the most and least abundant taxa.
3. Results
(a). Diversification rate analysis
Net diversification rate-through-time, mean phylorate, maximum shift credibility and best shift configuration plots show no clear tree-wide changes in diversification rate associated with the K/Pg. Many trees show relatively consistent increases in diversification rate towards the present, a likely artefact of the ‘pull of the present’ (electronic supplementary material, figures S1–S3; [67]). In Cypriniformes and Characiformes, subclades show shifts to an increased diversification rate on relatively short post-K/Pg branches with a subsequent gradual decrease in diversification rate approaching the present (figure 1). In Cypriniformes (figure 1a ), two clades show this signal: (Xenocyprididae, ((Acheilognathidae, Gobionidae), ((Tincidae, Tanichthyidae), Leuciscidae))) (hereafter ‘Cypriniformes A’), and a clade composed of all Cyprinidae except Probarbinae (hereafter, ‘Cypriniformes B’). In Characiformes, one clade shows a similar pattern (figure 1b ): this ‘Characiformes A’ clade includes all subgroups of Characidae besides Spintherobolinae.
Figure 1.
(a) Maximum shift credibility tree for Cypriniformes BAMM analysis with prior for expected shifts of 10. (b) Maximum shift credibility tree for Characiformes BAMM analysis with prior for expected shifts of 10. In (a) and (b), red circles indicate rate shifts and the red line indicates K/Pg boundary (66.021 Ma) [24].
In Siluriformes, a basal shift that includes all Loricariidae except Delturinae and Rhinelepinae is followed in some analyses by a rate decrease approaching the present, but in other analyses, the post-shift rate is nearly constant (electronic supplementary material, figure S4). This constancy suggests that the initial shift may also be a ‘pull of the present’ artefact (see Discussion). We thus note a possible Siluriformes signal but do not discuss it further.
In Cypriniformes B and Characiformes A, the inferred diversification rate increase is separated from the K/Pg by one branching event, but its proximity to the boundary is intriguing. There are similar inferred shifts on the stem of Mastacembelidae within Anabantaria and on the stem of Cichlidae in Ovalentaria (electronic supplementary material, figure S5). However, these shifts are on long internal branches (>30 Ma), complicating their relationship with the K/Pg. An additional shift occurs in Ovalentaria on the stem of a clade including the predominantly marine Blenniiformes, but moves between clades in different analyses and is sometimes reconstructed only on the stem of a blenniiform subclade. This shift is also reconstructed in the Acanthomorpha analysis; see below for other shifts in marine acanthomorphs.
The shift at the base of Characiformes A is recovered regardless of prior values for the expected number of shifts. With that prior set to 1, a rate shift is inferred on the stem of the most exclusive clade that includes both Cypriniformes A/B, but there are no shifts on the stems of either of those clades. In analyses with higher prior values (10 and 100), the posterior distribution centres on approximately 20 shifts, suggesting that setting the prior to 1, wherein the posterior distribution is centred on approximately 13 shifts, underestimates the number of shifts supported by the tree. The inferred rate shifts for both Cypriniformes A and B were recovered whether Raiamas intermedius and Rhodeus sinensis were included or not, but we focus on results where they were excluded.
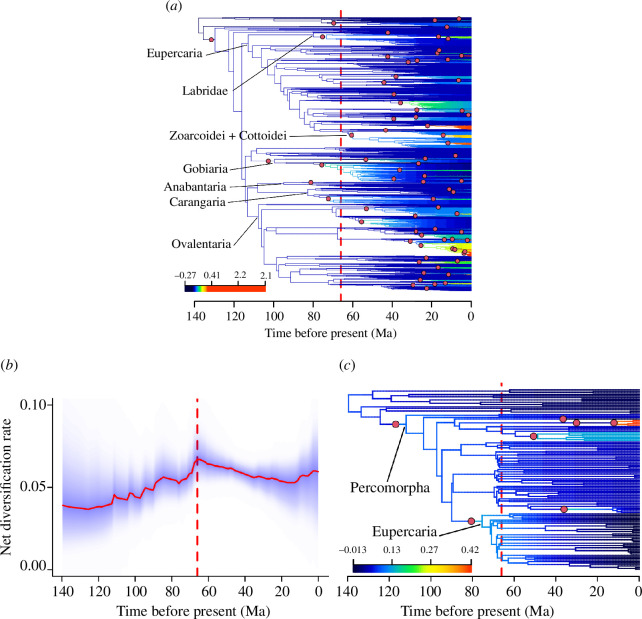
BAMM analyses for Acanthomorpha do not recover tree-wide diversification shifts associated with the K/Pg (figure 2a ), in contrast with previous findings [19]. There are inferred shifts on the stems of predominantly marine clades, some of which include long internal branches, but a few do not. The latter entails the shift noted above including or nested within Blenniiformes, a clade that includes both Zoarcoidei and Cottoidei, a subclade in Carangaria that includes many flatfishes and a subclade of Labridae. A subclade of the mixed freshwater and marine Gobiiformes also shows a signal, but the initial divergence of the clade is dated to approximately 74 Ma. These signals may support an underlying K/Pg influence but further analysis is beyond the scope of this study.
Figure 2.
(a) Maximum shift credibility tree for Acanthomorpha BAMM analysis with prior for expected shifts of 100. The scale is set to highlight changes in moderate levels of diversification rate. For more details of inferred shifts in Ovalentaria, see electronic supplementary material, figure S5. (b) Net diversification rate-through-time plot for Acanthomorpha BAMM analysis using data from A18 [19] with the prior for expected shifts of 10. (c) Maximum shift credibility tree for Acanthomorpha BAMM analysis using data from A18 with prior for expected shifts of 10. In (a) and (c), red circles indicate rate shifts. In (a), (b) and (c), the red line indicates the K/Pg boundary (66.021 Ma) [24].
The analysis of the phylogenetic tree from A18 reconstructs a spike in diversification rate across the K/Pg (figure 2b ), whereas phylorate plots from most analyses show no K/Pg-associated shifts in diversification rate. In some analyses, there is one rate increase at the base of Eupercaria (approx. 75.383 Ma; figure 2c ), followed by a gradual rate decrease approaching the present. This signal is consistent with a K/Pg effect but pre-dates the event by approximately 10 Ma. Divergence dates of five major percomorph clades, including Eupercaria (figure 3), show that correspondence of those dates with the K/Pg event in A18 is not recovered in the data of R18.
Figure 3.
(a) Acanthomorpha phylogeny from A18 [19] and (b) R18 [29]. Trees have been subsampled to include only taxa that are part of each phylogeny. Numbers in parentheses are divergence dates in Ma before present. In the R18 tree, the earliest diverging clade in Ovalentaria is Mugiliformes, but that clade is not in the A18 tree. The divergence date for Ovalentaria in the R18 tree is for Ovalentaria including Mugiliformes. Without Mugiliformes, the divergence date is 106.091 Ma.
(b). Morphotype analysis
We characterized the majority of centra (n = 245) to one of 15 morphotype groups (figure 4) and to an additional category that represented unique morphologies (n = 12). The remaining 94 centra were identified as caudal (n = 41), first centra (n = 24) or unidentifiable due to poor preservation (n = 29). Descriptions for all morphotypes and unique specimens are included in the electronic supplementary material.
Figure 4.
Representative specimens of morphotypes. Each specimen in dorsal, right lateral and ventral views, anterior to right; letters denote morphotype. The description of morphotype groups is in the electronic supplementary material. (a) DMNH EPV.143127, (b) DMNH EPV.121294, lateral reflected. (c) DMNH EPV.108389. (d) DMNH EPV.121287. (e) DMNH EPV.102871, lateral reflected. (f) DMNH EPV.107931. (g) DMNH EPV.108384, lateral reflected. (h) DMNH EPV.108407, lateral reflected. (j) DMNH EPV.99563, directionality estimated. (k) DMNH EPV.109606, lateral reflected. (l) DMNH EPV.102844, lateral reflected. (m) DMNH EPV.102852. (n) DMNH EPV.57436. (p) DMNH EPV.121291. (q) DMNH EPV.107927. Scale bar = 1 mm.
The morphotype analysis showed little evidence of K/Pg extinction (figure 5a ). Only groups P and Q are present in DMNH 3648 but not DMNH 2560. Three groups (J, L and M) are not present in DMNH 3648 but present in DMNH 2560, suggesting that the taxa represented by these morphotypes either immigrated to DMNH 2560, evolved from endemic groups present before the boundary or were simply not sampled. Notable changes in abundance between the two sites are present. The most abundant DMNH 2560 morphotype (D, n = 62) is represented by only one specimen from DMNH 3648. A and F both decrease in abundance across the boundary. All disappearances and appearances are in groups with low sample sizes (n < 6). Most unique morphologies were from DMNH 3648 (n = 8), with 4 from DMNH 2560 (figure 5a ).
Figure 5.
Results from morphotype analysis. (a) Relative abundance of morphotypes and (b) diversity indices between DMNH 3648 and DMNH 2560.
Diversity comparisons between DMNH 3648 and 2560 produced a significantly higher Simpson’s index (p = 0.0002), significantly lower Berger–Parker index (p = 0.0006) and significantly lower equitability (p = 0.0056) after the K/Pg (figure 5b ; table 1), suggesting that a small number of morphotypes dominated the post-K/Pg community. We assessed whether this pattern was driven primarily by the high relative abundance of morphotype D by comparing diversity indices with morphotype D excluded and found that the difference in both Simpson’s index (p = 0.0088) and the Berger–Parker index (p = 0.0238; table 1) remained significant, but the difference in equitability did not. We also tested the influence of the number of unique Cretaceous morphotypes, but when these are excluded, differences in both dominance indices and equitability remain significant, and evenness is significantly higher for DMNH 3648 (p = 0.0046; table 1). Hutcheson’s t-test shows that diversity (as calculated by the Shannon diversity index) was significantly higher (p = 0.0001) in the DMNH 3648 sample (table 1). This difference remained significant when morphotype D or the unique morphotypes were excluded. The difference in variance of the relative abundance data between the two sites was not significant using Levene’s test.
Table 1.
Statistical results from comparison between DMNH 3648 and DMNH 2560. The p-values for diversity indices are from permutation in Past4 software.
| primary analysis | |||
|---|---|---|---|
| test statistic | d.f. | p-value | |
| Hutcheson’s t‐test | 3.9216 | 167.52 | 0.0001 |
| Levene’s test | 0.3745 | 1, 35 | 0.5445 |
| index | DMNH 3648 | DMNH 2560 | |
| evenness | 0.7277 | 0.4674 | 0.0866 |
| equitability | 0.8939 | 0.7316 | 0.0056 |
| Berger–Parker | 0.1667 | 0.3584 | 0.0006 |
| Simpson | 0.9088 | 0.8066 | 0.0002 |
| unique morphotypes excluded | |||
|---|---|---|---|
| test statistic | d.f. | p-value | |
| Hutcheson’s t‐test | 2.508 | 173.2 | 0.0131 |
| Levene’s test | 0.0959 | 1,23 | 0.7596 |
| index | DMNH 3648 | DMNH 2560 | |
| evenness | 0.8293 | 0.5486 | 0.0046 |
| equitability | 0.9247 | 0.7659 | 0.0016 |
| Berger–Parker | 0.1875 | 0.3669 | 0.0041 |
| Simpson | 0.8844 | 0.7973 | 0.0028 |
| morphotype D excluded | |||
|---|---|---|---|
| test statistic | d.f. | p-value | |
| Hutcheson’s t‐test | 2.6331 | 167.77 | 0.0093 |
| Levene’s test | 0.3752 | 1,33 | 0.5444 |
| index | DMNH 3648 | DMNH 2560 | |
| evenness | 0.7334 | 0.5692 | 0.1536 |
| equitability | 0.8947 | 0.7967 | 0.0578 |
| Berger–Parker | 0.169 | 0.3153 | 0.0238 |
| Simpson | 0.9062 | 0.8385 | 0.0088 |
4. Discussion
(a). Diversification rate analysis
The lack of extinct species in an extant tree subjects it to the ‘pull of the present’, whereby recent cladogenesis is more likely to produce sampled descendants than deeper events [67]. Diversification in most clades studied here is consistent with this expectation, exhibiting diversification rates that either increase towards the present or are temporally stable. Among the exceptions are three highly diverse clades nested in Cypriniformes and Characiformes that collectively represent at least 11% of extant actinopterygian diversity (from FishBase [35–37]). These clades are characterized by an initial increase in diversification rate along their stem, a crown divergence within the first 15 Ma post-K/Pg and a gradual subsequent decrease in diversification rate. This signal is consistent with a scenario wherein the K/Pg event itself opens ecological opportunity which then permits a lineage to experience a burst of diversification.
Support for a K/Pg-induced diversification is not equally strong across the three clades. Cypriniformes A represents the strongest inference, with a stem that crosses the K/Pg and an initial divergence of 58.61 Ma. With Cypriniformes B and Characiformes A, the inferred shift is separated from the K/Pg by one branching event. Two points associate these diversifications with the K/Pg. First, all diversification dates are subject to uncertainty [8], and thus the immediate stem of each clade may have extended across the boundary rather than appearing just after it. Second, branching events are the data BAMM uses to reconstruct rate shifts. With only one speciation event separating the crowns of Cypriniformes B and Characiformes A from the K/Pg, limited data support the precise placement of a rate shift. In both cases, a rate shift may have occurred on the branch crossing the K/Pg, rather than on the stems of Cypriniformes B and Characiformes A. This logic could, of course, go both ways. Characiform diversification inferred using different data and a different tree also reconstructs a stem increase in diversification rate for Characiformes A [68], corroborating our results. No parallel studies exist for cypriniforms.
In contrast with previous results [19], we found no significant post-K/Pg diversification of Acanthomorpha. The relevant plot of net diversification rate in the A18 tree shows an increase across the K/Pg, but this spike is driven by a diversification increase on the Early Cretaceous stem of Percomorpha [69]. This percomorph increase is magnified (figure 2b ) in the calculation of the average acanthomorph diversification rate by subsequent crown divergences of several clades within Percomorpha relative to the low diversification rate outside Percomorpha. This relational increase produces the apparent spike in net diversification and marks a subtle but critical distinction to be aware of when working with BAMM. Multiple percomorph clades (Syngnatharia, Pelagiaria, Ovalentaria and Carangaria) with initial divergences near the K/Pg lack evidence of rate shifts along their respective stems. This suggests that their current diversity reflects a relatively constant diversification rate regime that is hidden in the net diversification rate-through-time plot. The tendency for these plots to be primarily driven not by rate shifts, but by the relative representation of taxa with high or low rates, complicates their use for inferring diversification trends. The coincidental divergence of Syngnatharia, Pelagiaria, Eupercaria, Ovalentaria and Carangaria with the K/Pg in the A18 tree is not recovered in the tree from R18 or in other studies of Acanthomorpha [6]. In our analysis of the A18 data, only Eupercaria shows a signal clearly consistent with an abrupt acceleration in diversification; however, the Eupercaria divergence pre-dates the K/Pg event by approximately 10 Ma. This divergence is actually older in the R18 tree, casting additional doubt on any widespread K/Pg effect on acanthomorph diversification. This doubt does not extend to the conclusion that the clade may have undergone significant post-K/Pg morphological diversification [6,18].
Clear signals of post-K/Pg diversification characterize only a small number of freshwater actinopterygian lineages in our analysis. At least some extinction signal, however, is likely to be lost given our use of the extant tree for BAMM analysis [31], and some extinction scenarios are unlikely to be captured in tree structure. For example, because the extant tree is the product of diversification—the combined outcome of extinction and speciation—a near zero change in diversification rate may belie a rise in both speciation and extinction rates. We may only expect a signal if one clade fills a post-extinction ecological opportunity. This scenario seems to characterize such widely recognized ‘winners’ of the K/Pg extinction as placental mammals [70] and birds [9], both of which radiated in the absence of non-avian dinosaurs [71]. If freshwater extinctions were less intense than those of the land or the ocean, then most clades might have suffered little across the boundary, re-expanding into habitats from a few survivors but with little total extirpation. Identified exceptions may reflect instances where total extirpation of other lineages was more likely, with survivors taking advantage of available habitat. Alternatively, if there were post-K/Pg shifts, they may be difficult to detect because of the relative dearth of articulated and diagnosable fossils of freshwater fish from K/Pg sediments [72]. While the R18 tree does include 130 calibrated nodes, these are spread throughout the immensity of the actinopterygian tree. Even if extinction was significant in fresh water, a lack of calibration near the K/Pg could smooth patterns of diversification such that they are less likely to be detected as distinct shifts. Finally, diversification may be a rare, immediate outcome of any extinction event, with morphological adaptation being the primary response [28] (e.g. [6]). These complications clarify the importance of fossils for building a detailed understanding of extinction.
(b). Morphotype analysis
Our data indicate a K/Pg loss of rare morphotypes, with the Palaeocene community dominated by relatively few morphotypes. Rare taxa are the most likely victims of any biodiversity crisis [73,74], including the current one [75], with losses opening ecological opportunities for survivors. At West Bijou, for example, the low Cretaceous abundance of morphotype D gave way to its relatively high Palaeocene representation. The approximately 128 kyr separating DMNH 2560 from the K/Pg was evidently not enough time for a full recovery to Cretaceous diversity levels. This is not a long interval, geologically speaking, but becomes more significant if it is a harbinger of recovery times for freshwater communities currently in crisis.
The change at West Bijou, from a diverse ecosystem to one dominated by fewer, highly abundant morphotypes is a familiar pattern. Communities that coalesce after extinction events, i.e. recovery faunas [76], tend to be low-diversity, low-evenness assemblages that opportunistically ‘take over’ after perturbation [77] and are dominated by relatively few bloom taxa [78]. Many such faunas have been identified; for example, marine invertebrates (Permo-Triassic) [79], mammals (K/Pg) [56] and planktonic foraminifera (K/Pg) [80]. These short-lived assemblages are eventually replaced by communities more similar to those that pre-date the perturbation [76]. A recovery fauna at West Bijou highlights how extinction and recovery play out in a local community.
Consistent with global reviews (e.g. [21]), studies of North American fossils conclude that inclusive freshwater fish taxa were relatively unaffected by the K/Pg but that local extirpations were likely [48,73]. The highly circumscribed nature of our fossil data, both geographically and temporally, complicates their relevance for global patterns but grants them an unprecedented ability to reveal localized dynamics. The West Bijou fossils serve as convincing evidence that, whether it be from the bolide itself or climatic perturbation [81], the K/Pg left its signature on local communities of freshwater fish. Increasing the phylogenetic resolution of the abundant fossil material (e.g. [55]) is an important next step in testing and expanding our conclusions.
(c). Diversity patterns in fresh water across the K/Pg
The K/Pg led to the diversification of several now-dominant clades of freshwater fish but had seemingly little effect on many others. At a community level, the event reduced diversity for more than 100 kyr after the boundary. Extrapolating the West Bijou data, one envisions a landscape of localized shifts in species abundance, perhaps including extirpation but not necessarily so, with little effect on broad-based phylogenetic diversification. These shifts in community structure represented opportunities for less-affected species to expand their local representation, as in morphotype D, or even to radiate, as with Cypriniformes A/B and Characiformes A.
Cypriniformes A/B and Characiformes A represent significant extant biodiversity, but it is unclear what (if anything) allowed them to differentially diversify within the niche space opened by the K/Pg [82]. Attempts to identify such diversity drivers often require ad hoc adaptationist scenarios of questionable heuristic value (see [83]). Unlike those clades wherein few species crossed the boundary (e.g. birds [9]), freshwater fish probably encompass hundreds of surviving lineages. We advocate that a stochastic driver is the preferred null hypothesis [82] with Cypriniformes A/B and Characiformes A diversifying post-K/Pg simply due to good fortune.
(d). Implications of freshwater extinction across the K/Pg
Seemingly insignificant shifts in local diversity like those at West Bijou may underlie long-term patterns in diversity and habitat dominance. Extinction is often measured at the species level, despite the finding that changes in relative abundance at the population level can severely impact ecosystem function and count among the most important biodiversity losses [84]. Although there is evidence that freshwater ecosystems were buffered to some degree from the K/Pg event [73,85,86], local communities registered the impact. Freshwater communities may not be as fortunate in the current crisis, where anthropogenic processes have unique effects on freshwater habitats (e.g. nitrogenous waste, dams, irrigation; [87]). We should expect these local effects to have intense and widespread influences on freshwater diversity. The fossil analysis shows that at local scales, recovery to pre-perturbation diversity levels may happen eventually, but will take far longer than one or even a dozen human lifetimes. Even with recovery, the phylogenetic distribution of freshwater diversity may be irreversibly changed. If an upside exists, it is that we are still relatively early in the sixth mass extinction, and through expanded investigations of how local diversity changes with environmental perturbation and by integrating what we learn from unique palaeontological sites like West Bijou, we will be better situated to respond to the conservation challenges of freshwater lineages and their communities.
5. Conclusions
It is difficult to disentangle the long-held idea that freshwater fish communities were relatively stable across the K/Pg mass extinction from the poor quality of the relevant fossil record. Here, we use a two-pronged approach to assess K/Pg extinction and recovery dynamics in freshwater fish at both global and local scales. Our approach uses diversification rate analysis of molecular phylogenies of major freshwater clades and morphotype analysis of a unique set of microsites in the Denver Basin to test the hypothesis that freshwater actinopterygians were unaffected by the K/Pg. Our findings expand our understanding of the extinction landscape across the K/Pg by providing evidence that several highly diverse extant clades experienced initial bursts of post-event diversification. Fossil analysis indicates that these global patterns of diversification were driven by stochastic localized extinction and faunal turnover. Our study demonstrates that the K/Pg extinction not only had effects on freshwater actinopterygian communities but that those effects were detectable approximately 128 thousand years after the event. Ultimately, post-K/Pg bursts of diversification underlie the present diversity of several dominant freshwater clades. These patterns provide a new perspective that is particularly important in light of the current biodiversity crisis and the dire conservation challenges it presents. The K/Pg dynamics of freshwater actinopterygians suggests that the current crisis in freshwater communities is likely to produce long-lasting effects, certainly when considered on the scale of human history and civilization.
Acknowledgements
We thank the staff and volunteers of the Denver Museum of Nature and Science, particularly C. Nelson for picking the West Bijou material and K. Mackenzie for collections support. We are also grateful for the generous support of M. L. and S. R. Kneller. The Savory Institute and B. Shelton provided critical access to the West Bijou Creek area. We thank A. Balanoff, D. Brinkman, S. Cooke, D. Cerio, W. Foster, P. Gensbigler and F. Torres for helpful discussions. Two anonymous reviewers and an associate editor greatly improved the manuscript with their comments and suggestions.
Contributor Information
Jacob D. Wilson, Email: jwils183@jhmi.edu.
E. J. Huang, Email: yhuan155@jhmi.edu.
Tyler R. Lyson, Email: Tyler.Lyson@dmns.org.
Gabriel S. Bever, Email: gbever1@jhmi.edu.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
All data supporting this study are uploaded to Dryad [88].
Supplementary material is available online [89].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
J.D.W.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, resources, software, validation, visualization, writing—original draft, writing—review and editing; E.J.H.: conceptualization, data curation, formal analysis, investigation, methodology, software, validation, writing—review and editing; T.R.L.: conceptualization, data curation, funding acquisition, investigation, methodology, resources, supervision, validation, writing—review and editing; G.S.B.: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
Fieldwork funding was provided by M. L. and S. R. Kneller to T.R.L. This work was also funded in part by grants to J.D.W. from the Paleontological Society and the Explorers Club and to T.R.L. by NSF-FRES-2317666.
References
- 1. Cuvier G. 1796. Mémoire sur les espèces d’eléphans tant vivantes que fossiles, lu à la séance publique de l’Institut national le 15 germinal, an IV. Mag. encyclop. 2 , 440–445. [Google Scholar]
- 2. Rudwick MJS. 1997. Georges cuvier, fossil bones, and geological catastrophes. Chicago, IL: University of Chicago Press. ( 10.7208/chicago/9780226731087.001.0001) [DOI] [Google Scholar]
- 3. Wilson EO. 1994. Biodiversity: challenge, science, opportunity. Am. Zool. 34 , 5–11. ( 10.1093/icb/34.1.5) [DOI] [Google Scholar]
- 4. Barnosky AD, et al. 2011. Has the earth’s sixth mass extinction already arrived? Nature 471 , 51–57. ( 10.1038/nature09678) [DOI] [PubMed] [Google Scholar]
- 5. Ceballos G, Ehrlich PR, Barnosky AD, García A, Pringle RM, Palmer TM. 2015. Accelerated modern human-induced species losses: entering the sixth mass extinction. Sci. Adv. 1 , e1400253. ( 10.1126/sciadv.1400253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghezelayagh A, et al. 2022. Prolonged morphological expansion of spiny-rayed fishes following the end-cretaceous. Nat. Ecol. Evol. 6 , 1211–1220. ( 10.1038/s41559-022-01801-3) [DOI] [PubMed] [Google Scholar]
- 7. Bertrand OC, et al. 2022. Brawn before brains in placental mammals after the end-cretaceous extinction. Science 376 , 80–85. ( 10.1126/science.abl5584) [DOI] [PubMed] [Google Scholar]
- 8. Donoghue PCJ, Yang Z. 2016. The evolution of methods for establishing evolutionary timescales. Phil. Trans. R. Soc. B 371 , 20160020. ( 10.1098/rstb.2016.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Field DJ, Benito J, Chen A, Jagt JWM, Ksepka DT. 2020. Late cretaceous neornithine from Europe illuminates the origins of crown birds. Nature 579 , 397–401. ( 10.1038/s41586-020-2096-0) [DOI] [PubMed] [Google Scholar]
- 10. Lee MSY, Ho SYW. 2016. Molecular clocks. Curr. Biol. 26 , R399–402. ( 10.1016/j.cub.2016.03.071) [DOI] [PubMed] [Google Scholar]
- 11. Newbrey MG, Bozek MA. 2000. A new species of joffrichthys (Teleostei: Osteoglossidae) from the sentinel butte formation, (Paleocene) of North Dakota, USA. J. Vertebr. Paleontol. 20 , 12–20. ( 10.1671/0272-4634(2000)020[0012:ANSOJT]2.0.CO;2) [DOI] [Google Scholar]
- 12. Murray AM, Brinkman DB, Newbrey MG, Neuman AG. 2020. Earliest north American articulated freshwater acanthomorph fish (teleostei: percopsiformes) from upper cretaceous deposits of Alberta, Canada. Geol. Mag. 157 , 1087–1096. ( 10.1017/S0016756819001328) [DOI] [Google Scholar]
- 13. Hilton EJ, Grande L. 2023. Late cretaceous sturgeons (Acipenseridae) from North America, with two new species from the tanis site in the hell creek formation of North Dakota. J. Paleontol. 97 , 189–217. ( 10.1017/jpa.2022.81) [DOI] [Google Scholar]
- 14. Brownstein CD, Lyson TR. 2022. Giant gar from directly above the cretaceous-palaeogene boundary suggests healthy freshwater ecosystems existed within thousands of years of the asteroid impact. Biol. Lett. 18 , 20220118. ( 10.1098/rsbl.2022.0118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hibbard CW. 1949. Techniques of collecting microvertebrate fossils. Contrib. Mus. Paleontol. Univ. Mich. 8 , 7–19. [Google Scholar]
- 16. Newbrey MG, Murray AM, Wilson MVH, Brinkman DB, Neuman AG. 2009. Seventy-five-million-year-old tropical tetra-like fish from Canada tracks cretaceous global warming. Proc. R. Soc. B 276 , 3829–3833. ( 10.1098/rspb.2009.1047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Friedman M. 2009. Ecomorphological selectivity among marine teleost fishes during the end-cretaceous extinction. Proc. Natl Acad. Sci. USA 106 , 5218–5223. ( 10.1073/pnas.0808468106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Friedman M. 2010. Explosive morphological diversification of spiny-finned teleost fishes in the aftermath of the end-cretaceous extinction. Proc. R. Soc. B 277 , 1675–1683. ( 10.1098/rspb.2009.2177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alfaro ME, Faircloth BC, Harrington RC, Sorenson L, Friedman M, Thacker CE, Oliveros CH, Černý D, Near TJ. 2018. Explosive diversification of marine fishes at the cretaceous-palaeogene boundary. Nat. Ecol. Evol. 2 , 688–696. ( 10.1038/s41559-018-0494-6) [DOI] [PubMed] [Google Scholar]
- 20. Friedman M, Sallan LC. 2012. Five hundred million years of extinction and recovery: a phanerozoic survey of large‐scale diversity patterns in fishes. Palaeontology 55 , 707–742. ( 10.1111/j.1475-4983.2012.01165.x) [DOI] [Google Scholar]
- 21. Guinot G, Cavin L. 2016. ‘Fish’ (Actinopterygii and Elasmobranchii) diversification patterns through deep time. Biol. Rev. Camb. Philos. Soc. 91 , 950–981. ( 10.1111/brv.12203) [DOI] [PubMed] [Google Scholar]
- 22. Bryant LJ. 1989. Non-dinosaurian lower vertebrates across the Cretaceous–Tertiary boundary, northeastern Montana. Berkeley, CA: University of California Press. [Google Scholar]
- 23. Barclay RS, Johnson KR. 2004. West Bijou site Cretaceous–Tertiary boundary, Denver Basin, Colorado. In GSA field guide 5: field trips in the Southern Rocky Mountains, USA (eds Nelson EP, Erslev EA), pp. 59–68. Boulder, CO: Geological Society of America. ( 10.1130/0-8137-0005-1.59) [DOI] [Google Scholar]
- 24. Clyde WC, Ramezani J, Johnson KR, Bowring SA, Jones MM. 2016. Direct high-precision U–pb geochronology of the end-cretaceous extinction and calibration of paleocene astronomical timescales. Earth Planet. Sci. Lett. 452 , 272–280. ( 10.1016/j.epsl.2016.07.041) [DOI] [Google Scholar]
- 25. Dahlberg EL, Eberle JJ, Sertich JJW, Miller IM. 2016. A new earliest paleocene (puercan) mammalian fauna from Colorado’s Denver basin, USA. Rocky. Mountain. Geol. 51 , 1–22. ( 10.2113/gsrocky.51.1.1) [DOI] [Google Scholar]
- 26. Hull P. 2015. Life in the aftermath of mass extinctions. Curr. Biol. 25 , R941–52. ( 10.1016/j.cub.2015.08.053) [DOI] [PubMed] [Google Scholar]
- 27. Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press. ( 10.1093/oso/9780198505235.001.0001) [DOI] [Google Scholar]
- 28. Hedges SB, Marin J, Suleski M, Paymer M, Kumar S. 2015. Tree of life reveals clock-like speciation and diversification. Mol. Biol. Evol. 32 , 835–845. ( 10.1093/molbev/msv037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rabosky DL, et al. 2018. An inverse latitudinal gradient in speciation rate for marine fishes. Nature 559 , 392–395. ( 10.1038/s41586-018-0273-1) [DOI] [PubMed] [Google Scholar]
- 30. Rabosky DL. 2014. Automatic detection of key innovations, rate shifts, and diversity-dependence on phylogenetic trees. PLoS One 9 , e89543. ( 10.1371/journal.pone.0089543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rabosky DL. 2010. Extinction rates should not be estimated from molecular phylogenies. Evolution. 64 , 1816–1824. ( 10.1111/j.1558-5646.2009.00926.x) [DOI] [PubMed] [Google Scholar]
- 32. Sun M, Folk RA, Gitzendanner MA, Soltis PS, Chen Z, Soltis DE, Guralnick RP. 2020. Estimating rates and patterns of diversification with incomplete sampling: a case study in the rosids. Am. J. Bot. 107 , 895–909. ( 10.1002/ajb2.1479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paradis E, Schliep K. 2019. Ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35 , 526–528. ( 10.1093/bioinformatics/bty633) [DOI] [PubMed] [Google Scholar]
- 34. Betancur-R R, Ortí G, Pyron RA. 2015. Fossil-based comparative analyses reveal ancient marine ancestry erased by extinction in ray-finned fishes. Ecol. Lett. 18 , 441–450. ( 10.1111/ele.12423) [DOI] [PubMed] [Google Scholar]
- 35. Welcomme RL. 1988. International introductions of inland aquatic species. Rome, Italy: Food and Agricultural Organization of the United Nations. [Google Scholar]
- 36. Houde ED, Zastrow CE. 1993. Ecosystem- and taxon-specific dynamic and energetics properties of larval fish assemblages. Bull. Mar. Sci. 53 , 290–335. [Google Scholar]
- 37. Froese R, Pauly D. FishBase 2000: concepts, design and data sources. Los Baños, CA: ICLARM. [Google Scholar]
- 38. Boettiger C, Lang DT, Wainwright PC. 2012. Rfishbase: exploring, manipulating and visualizing fishbase data from R. J. Fish Biol. 81 , 2030–2039. ( 10.1111/j.1095-8649.2012.03464.x) [DOI] [PubMed] [Google Scholar]
- 39. Rabosky DL, Grundler M, Anderson C, Title P, Shi JJ, Brown JW, Huang H, Larson JG. 2014. bammtools: an R package for the analysis of evolutionary dynamics on phylogenetic trees. Methods Ecol. Evol. 5 , 701–707. ( 10.1111/2041-210X.12199) [DOI] [Google Scholar]
- 40. Plummer M, Best N, Cowles K, Vines K. 2006. CODA: convergence diagnosis and output analysis for MCMC. R. News 6 , 7–11. [Google Scholar]
- 41. Patterson C, Rosen DE. 1977. Review of ichthyodectiform and other mesozoic teleost fishes and the theory and practice of classifying fossils. Bull. Am. Mus. Nat. Hist. 158 , 83–172. [Google Scholar]
- 42. Bever GS. 2005. Variation in the ilium of north American Bufo (Lissamphibia; Anura) and its implications for species-level identification of fragmentary anuran fossils. J. Vertebr. Paleontol. 25 , 548–560. ( 10.1671/0272-4634(2005)025[0548:VITION]2.0.CO;2) [DOI] [Google Scholar]
- 43. Bell CJ, Gauthier JA, Bever GS. 2010. Covert biases, circularity, and apomorphies: a critical look at the North American quaternary herpetofaunal stability hypothesis. Quat. Int. 217 , 30–36. ( 10.1016/j.quaint.2009.08.009) [DOI] [Google Scholar]
- 44. Longrich NR, Bhullar BAS, Gauthier JA. 2012. Mass extinction of lizards and snakes at the cretaceous-paleogene boundary. Proc. Natl Acad. Sci. USA 109 , 21396–21401. ( 10.1073/pnas.1211526110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brinkman DB, Neuman AG. 2002. Teleost centra from uppermost Judith River group (Dinosaur Park Formation, Campanian) of Alberta, Canada. J. Paleontol. 76 , 138–155. ( 10.1666/0022-3360(2002)0762.0.CO;2) [DOI] [Google Scholar]
- 46. Sinha S, Brinkman DB, Murray AM, Krause DW. 2021. Late paleocene fishes of the ravenscrag formation, roche percée area, Southeastern Saskatchewan, Canada. J. Vertebr. Paleontol. 41 , e1957907. ( 10.1080/02724634.2021.1957907) [DOI] [Google Scholar]
- 47. Brinkman DB, Newbrey MG, Neuman AG, Eaton JG. 2013. Freshwater osteichthyes from the Cenomanian to Late Campanian of Grand Staircase–Escalante national monument, Utah. In At the top of the Grand Staircase(eds Titus AL, Loewen MA), pp. 195–236. Bloomington, IN: Indiana University Press. [Google Scholar]
- 48. Brinkman DB, Divay JD, DeMar DG, Wilson Mantilla GP. 2021. A systematic reappraisal and quantitative study of the nonmarine teleost fishes from the late Maastrichtian of the western interior of North America: evidence from vertebrate microfossil localities. Can. J. Earth Sci. 58 , 936–967. ( 10.1139/cjes-2020-0168) [DOI] [Google Scholar]
- 49. Ash A, Ellis B, Hickey LJ, Johnson KR, Wilf P, Wing S. 1999. Manual of leaf architecture. Washington, DC: Smithsonian Institution. [Google Scholar]
- 50. Nichols DJ. 2002. Palynology and palynostratigraphy of the Hell Creek formation in North Dakota: a microfossil record of plants at the end of Cretaceous time. In The Hell Creek formation and the Cretaceous-Tertiary boundary in the northern great plains: an integrated continental record of the end of the Cretaceous (eds Hartman JH, Johnson KR, Nichols DJ), pp. 393–456. Boulder, CO: Geological Society of America. ( 10.1130/0-8137-2361-2.393) [DOI] [Google Scholar]
- 51. Nichols DJ, Fleming RF. 2002. Palynology and palynostratigraphy of Maastrichtian, Paleocene, and Eocene strata in the Denver Basin, Colorado. Rocky. Mt. Geol. 37 , 135–163. ( 10.2113/gsrocky.37.2.135) [DOI] [Google Scholar]
- 52. Barclay RS, Johnson KR, Betterton WJ, Dilcher DL. 2003. Stratigraphy and megaflora of a K-T boundary section in the eastern Denver Basin, Colorado. Rocky. Mountain. Geol. 38 , 45–71. ( 10.2113/gsrocky.38.1.45) [DOI] [Google Scholar]
- 53. Brinkman DB. 2008. The structure of late Cretaceous (late Campanian) nonmarine aquatic communities: a guild analysis of two vertebrate microfossil localities in Dinosaur Provincial Park, Alberta, Canada. In Vertebrate microfossil assemblages: their role in paleoecology and paleobiogeography(eds Sankey JT, Baszio S), pp. 33–60. Bloomington, IN: Indiana University Press. [Google Scholar]
- 54. Sinha S, Brinkman DB, Murray AM. 2019. A morphological study of vertebral centra in extant species of pike, esox (Teleostei: Esociformes). Vert. Anat. Morph. Palaeo. 7 , 111–128. ( 10.18435/vamp29357) [DOI] [Google Scholar]
- 55. Murray AM, Brinkman DB. Morphological variation in the first vertebra among acanthomorph fishes: a guide for identifying fossil centra from microvertebrate sites. Vert. Anat. Morph. Palaeo. 11 . ( 10.18435/vamp29392) [DOI] [Google Scholar]
- 56. Wilson GP. 2014. Mammalian extinction, survival, and recovery dynamics across the Cretaceous–Paleogene boundary in northeastern Montana, USA. In Through the end of the Cretaceous in the type locality of the Hell Creek formation in Montana and adjacent areas(eds Wilson GP, Clemens WA, Horner JR, Hartman JH), pp. 365–392. Boulder, CO: Geological Society of America. ( 10.1130/2014.2503(15) [DOI] [Google Scholar]
- 57. Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4 , 1–9. [Google Scholar]
- 58. Buzas MA, Gibson TG. 1969. Species diversity: benthonic foraminifera in western North Atlantic. Science 163 , 72–75. ( 10.1126/science.163.3862.72) [DOI] [PubMed] [Google Scholar]
- 59. Pielou EC. 1975. Ecological diversity. New York, NY: Wiley. [Google Scholar]
- 60. Simpson EH. 1949. Measurement of diversity. Nature 163 , 688–688. ( 10.1038/163688a0) [DOI] [Google Scholar]
- 61. Lande R. 1996. Statistics and partitioning of species diversity, and similarity among multiple communities. Oikos 76 , 5. ( 10.2307/3545743) [DOI] [Google Scholar]
- 62. Berger WH, Parker FL. 1970. Diversity of planktonic foraminifera in deep-sea sediments. Science 168 , 1345–1347. ( 10.1126/science.168.3937.1345) [DOI] [PubMed] [Google Scholar]
- 63. Gibson TG, Buzas MA. 1973. Species diversity: patterns in modern and miocene foraminifera of the eastern margin of North America. Geol. Soc. Am. Bull. 84 , 217–238. ( 10.1130/0016-7606(1973)842.0.CO;2) [DOI] [Google Scholar]
- 64. Magurran AE. 2004. Measuring biological diversity. Malden, MA: Blackwell. [DOI] [PubMed] [Google Scholar]
- 65. Hutcheson K. 1970. A test for comparing diversities based on the Shannon formula. J. Theor. Biol. 29 , 151–154. ( 10.1016/0022-5193(70)90124-4) [DOI] [PubMed] [Google Scholar]
- 66. Fox J, Weisberg S. 2019. An R companion to applied regression, 3rd edn. Thousand Oaks, CA: Sage. [Google Scholar]
- 67. Nee S, May RM, Harvey PH. 1994. The reconstructed evolutionary process. Phil. Trans. R. Soc. B 344 , 305–311. ( 10.1098/rstb.1994.0068) [DOI] [PubMed] [Google Scholar]
- 68. Melo BF, et al. 2021. Accelerated diversification explains the exceptional species richness of tropical characoid fishes. Syst. Biol. 71 , 78–92. ( 10.1093/sysbio/syab040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Alfaro ME, Santini F, Brock C, Alamillo H, Dornburg A, Rabosky DL, Carnevale G, Harmon LJ. 2009. Nine exceptional radiations plus high turnover explain species diversity in jawed vertebrates. Proc. Natl Acad. Sci. USA 106 , 13410–13414. ( 10.1073/pnas.0811087106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. O’Leary MA, et al. 2013. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science 339 , 662–667. ( 10.1126/science.1229237) [DOI] [PubMed] [Google Scholar]
- 71. Schulte P, et al. 2010. The chicxulub asteroid impact and mass extinction at the Cretaceous-Paleogene boundary. Science 327 , 1214–1218. ( 10.1126/science.1177265) [DOI] [PubMed] [Google Scholar]
- 72. Grande L, Grande T. 1999. A new species of †Notogoneus (Teleostei: Gonorynchidae) from the Upper Cretaceous Two Medicine formation of Montana, and the poor Cretaceous record of freshwater fishes from North America. J. Vertebr. Paleontol. 19 , 612–622. ( 10.1080/02724634.1999.10011175) [DOI] [Google Scholar]
- 73. Archibald JD, Bryant LJ. 1990. Differential Cretaceous/Tertiary extinctions of nonmarine vertebrates: evidence from northeastern Montana. In Global catastrophes in earth history: an interdisciplinary conference on impacts, volcanism, and mass mortality (eds Sharpton D, Ward PD, Sharpton VL, Ward PD), pp. 549–562. Geological Society of America: Boulder. ( 10.1130/SPE247-p549) [DOI] [Google Scholar]
- 74. McKinney ML. 1997. Extinction vulnerability and selectivity: combining ecological and paleontological views. Annu. Rev. Ecol. Syst. 28 , 495–516. ( 10.1146/annurev.ecolsys.28.1.495) [DOI] [Google Scholar]
- 75. Purvis A, Gittleman JL, Cowlishaw G, Mace GM. 2000. Predicting extinction risk in declining species. Proc. Biol. Sci. 267 , 1947–1952. ( 10.1098/rspb.2000.1234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Erwin DH. 1998. The end and the beginning: recoveries from mass extinctions. Trends Ecol. Evol. 13 , 344–349. ( 10.1016/s0169-5347(98)01436-0) [DOI] [PubMed] [Google Scholar]
- 77. Hull PM, Darroch SAF, Erwin DH. 2015. Rarity in mass extinctions and the future of ecosystems. Nature 528 , 345–351. ( 10.1038/nature16160) [DOI] [PubMed] [Google Scholar]
- 78. Hansen TA. 1988. Early tertiary radiation of marine molluscs and the long-term effects of the cretaceous-tertiary extinction. Paleobiology 14 , 37–51. ( 10.1017/S0094837300011787) [DOI] [Google Scholar]
- 79. Schubert JK, Bottjer DJ. 1995. Aftermath of the permian-triassic mass extinction event: paleoecology of lower triassic carbonates in the western USA. Palaeogeogr. Palaeoclimatol. Palaeoecol. 116 , 1–39. ( 10.1016/0031-0182(94)00093-N) [DOI] [Google Scholar]
- 80. Olsson RK, Hemleben C, Berggren WA, Liu C. 1992. Wall texture classification of planktonic foraminifera genera in the lower Danian. J. Foram. Res. 22 , 195–213. ( 10.2113/gsjfr.22.3.195) [DOI] [Google Scholar]
- 81. Lyson TR, et al. 2019. Exceptional continental record of biotic recovery after the Cretaceous-Paleogene mass extinction. Science 366 , 977–983. ( 10.1126/science.aay2268) [DOI] [PubMed] [Google Scholar]
- 82. Title PO, et al. 2024. The macroevolutionary singularity of snakes. Science 383 , 918–923. ( 10.1126/science.adh2449) [DOI] [PubMed] [Google Scholar]
- 83. Gould SJ, Lewontin RC. 1979. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc. R. Soc. B 205 , 581–598. ( 10.1098/rspb.1979.0086) [DOI] [PubMed] [Google Scholar]
- 84. Dirzo R, Ceballos G, Ehrlich PR. 2022. Circling the drain: the extinction crisis and the future of humanity. Phil. Trans. R. Soc. B 377 , 20210378. ( 10.1098/rstb.2021.0378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sheehan PM, Fastovsky DE. 1992. Major extinctions of land-dwelling vertebrates at the Cretaceous–Tertiary boundary, eastern Montana. Geology 20 , 556–560. ( 10.1130/0091-7613(1992)0202.3.CO;2) [DOI] [Google Scholar]
- 86. Hartman JH, Johnson KR, Nichols DJ. 2002. Patterns of geographic variation in latest Cretaceous vertebrates: evidence from the turtle component. In The Hell Creek formation and the Cretaceous–Tertiary boundary in the northern great plains: an integrated continental record of the end of the Cretaceous (eds Hartman H, Johnson KR, Nichols DJ), pp. 177–190. Boulder, CO: Geological Society of America. ( 10.1130/SPE361) [DOI] [Google Scholar]
- 87. Strayer DL, Dudgeon D. 2010. Freshwater biodiversity conservation: recent progress and future challenges. Freshw. Sci. 29 , 344–358. ( 10.1899/08-171.1) [DOI] [Google Scholar]
- 88. Wilson JD, Huang EJ, Lyson TR, Bever GS. 2024. Data for: Freshwater fish and the Cretaceous/Palaeogene boundary: a critical assessment of survivorship patterns. Dryad Digital Repository. ( 10.5061/dryad.v6wwpzh4d) [DOI] [PMC free article] [PubMed]
- 89. Wilson JD, Huang EJ, Lyson T, Bever GS. 2024. Data from: Freshwater fish and the Cretaceous/Palaeogene boundary: a critical assessment of survivorship patterns. Figshare. ( 10.6084/m9.figshare.c.7412352) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting this study are uploaded to Dryad [88].
Supplementary material is available online [89].




![(Left) Acanthomorpha phylogeny from A18 [19] and (right) R18 [29].](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/93b2/11352605/87de4d265d61/rspb.2024.1025.f003.jpg)

