Abstract
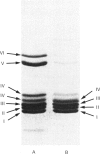
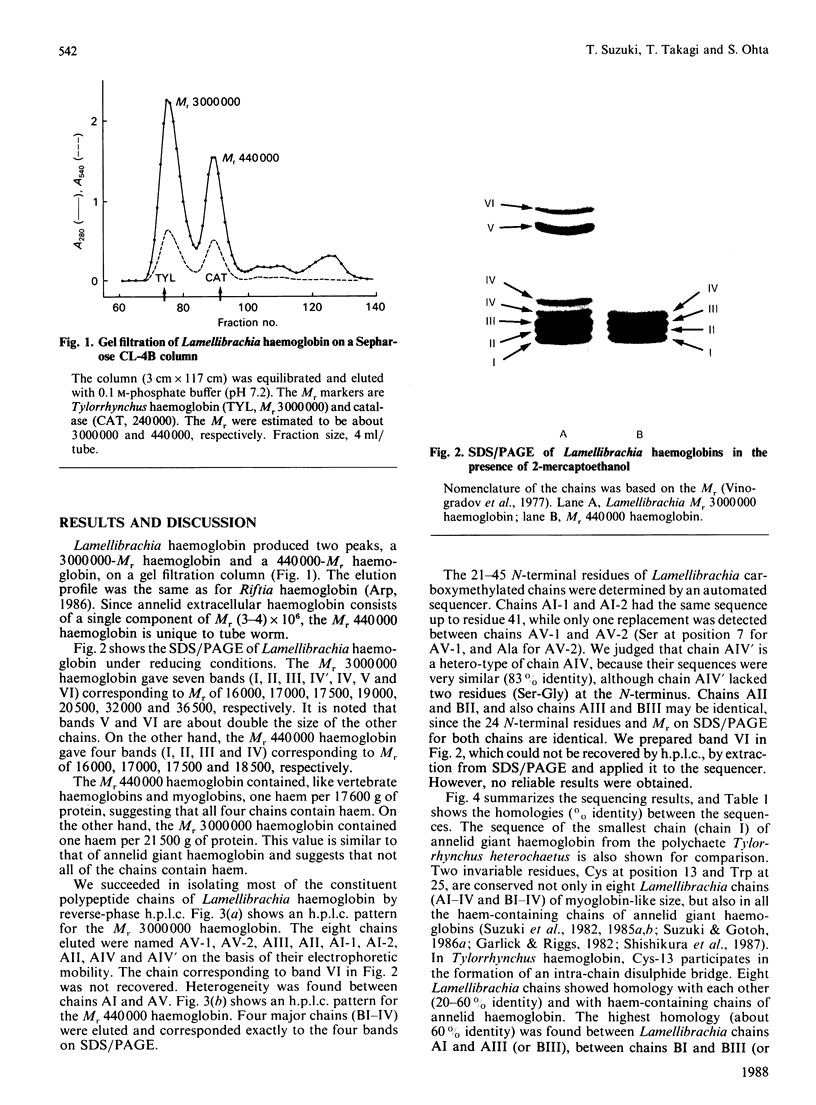
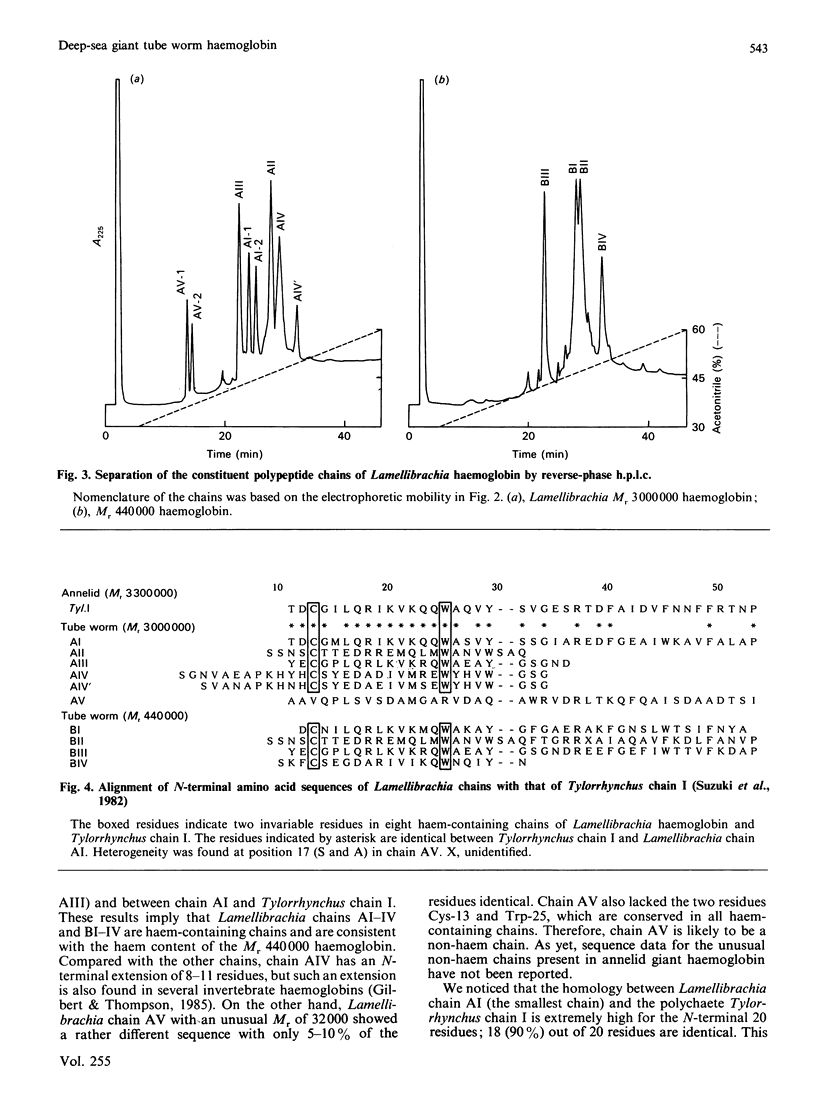
The deep-sea giant tube worm Lamellibrachia, belonging to the phylum Vestimentifera, contains two extracellular haemoglobins, an Mr 3,000,000 haemoglobin and an Mr 440,000 haemoglobin. The former has a hexagonal bilayer structure and consists of six polypeptide chains (AI-VI); a study of its haem content shows that not all of the chains contain haem. The Mr 440,000 haemoglobin consists of four haem-containing chains (BI-IV). We isolated most of the chains by reverse-phase chromatography and determined the amino acid sequences of the 21-45 N-terminal residues. Eight chains (AI-IV and BI-IV) showed significant homology with haem-containing chains of annelid giant haemoglobin. The highest homology was found between Lamellibrachia chain AI and Tylorrhynchus chain I; surprisingly, 18 out of the 20 N-terminal residues are identical. On the other hand, chain AV, with an unusual Mr of 32,000, showed a rather different sequence and is likely to be a non-haem chain which might act as a linker protein in the assembly of the haem-containing chains. From these results, we conclude that the tube worm Mr 3,000,000 haemoglobin is highly homologous with annelid haemoglobin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arp A. J., Childress J. J. Blood function in the hydrothermal vent vestimentiferan tube worm. Science. 1981 Jul 17;213(4505):342–344. doi: 10.1126/science.213.4505.342. [DOI] [PubMed] [Google Scholar]
- Arp A. J., Childress J. J. Sulfide Binding by the Blood of the Hydrothermal Vent Tube Worm Riftia pachyptila. Science. 1983 Jan 21;219(4582):295–297. doi: 10.1126/science.219.4582.295. [DOI] [PubMed] [Google Scholar]
- Corliss J. B., Dymond J., Gordon L. I., Edmond J. M., von Herzen R. P., Ballard R. D., Green K., Williams D., Bainbridge A., Crane K., van Andel T. H. Submarine thermal sprirngs on the galapagos rift. Science. 1979 Mar 16;203(4385):1073–1083. doi: 10.1126/science.203.4385.1073. [DOI] [PubMed] [Google Scholar]
- Garlick R. L., Riggs A. F. The amino acid sequence of a major polypeptide chain of earthworm hemoglobin. J Biol Chem. 1982 Aug 10;257(15):9005–9015. [PubMed] [Google Scholar]
- Gilbert A. T., Thompson E. O. Amino acid sequence of the beta-chain of the tetrameric haemoglobin of the bivalve mollusc, Anadara trapezia. Aust J Biol Sci. 1985;38(3):221–236. doi: 10.1071/bi9850221. [DOI] [PubMed] [Google Scholar]
- Goodman M., Moore G. W., Matsuda G. Darwinian evolution in the genealogy of haemoglobin. Nature. 1975 Feb 20;253(5493):603–608. doi: 10.1038/253603a0. [DOI] [PubMed] [Google Scholar]
- Jones M. L. Riftia pachyptila Jones: Observations on the Vestimentiferan Worm from the Galapagos Rift. Science. 1981 Jul 17;213(4505):333–336. doi: 10.1126/science.213.4505.333. [DOI] [PubMed] [Google Scholar]
- Mainwaring M. G., Lugo S. D., Fingal R. A., Kapp O. H., Vinogradov S. N. The dissociation of the extracellular hemoglobin of Lumbricus terrestris at acid pH and its reassociation at neutral pH. A new model of its quaternary structure. J Biol Chem. 1986 Aug 15;261(23):10899–10908. [PubMed] [Google Scholar]
- Shishikura F., Snow J. W., Gotoh T., Vinogradov S. N., Walz D. A. Amino acid sequence of the monomer subunit of the extracellular hemoglobin of Lumbricus terrestris. J Biol Chem. 1987 Mar 5;262(7):3123–3131. [PubMed] [Google Scholar]
- Suzuki T., Furukohri T., Gotoh T. Subunit structure of extracellular hemoglobin from the polychaete Tylorrhynchus heterochaetus and amino acid sequence of the constituent polypeptide chain (IIC). J Biol Chem. 1985 Mar 10;260(5):3145–3154. [PubMed] [Google Scholar]
- Suzuki T., Gotoh T. Subunit assembly of giant haemoglobin from the polychaete Tylorrhynchus heterochaetus. J Mol Biol. 1986 Jul 5;190(1):119–123. doi: 10.1016/0022-2836(86)90081-1. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Gotoh T. The complete amino acid sequence of giant multisubunit hemoglobin from the polychaete Tylorrhynchus heterochaetus. J Biol Chem. 1986 Jul 15;261(20):9257–9267. [PubMed] [Google Scholar]
- Suzuki T., Yasunaga H., Furukohri T., Nakamura K., Gotoh T. Amino acid sequence of polypeptide chain IIB of extracellular hemoglobin from the polychaete Tylorrhynchus heterochaetus. J Biol Chem. 1985 Sep 25;260(21):11481–11487. [PubMed] [Google Scholar]
- Vinogradov S. N., Lugo S. D., Mainwaring M. G., Kapp O. H., Crewe A. V. Bracelet protein: a quaternary structure proposed for the giant extracellular hemoglobin of Lumbricus terrestris. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8034–8038. doi: 10.1073/pnas.83.21.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S. N., Shlom J. M., Hall B. C., Kapp O. H., Mizukami H. The dissociation of Lumbricus terrestris hemoglobin: a model of its subunit structure. Biochim Biophys Acta. 1977 May 27;492(1):136–155. doi: 10.1016/0005-2795(77)90221-5. [DOI] [PubMed] [Google Scholar]