Abstract
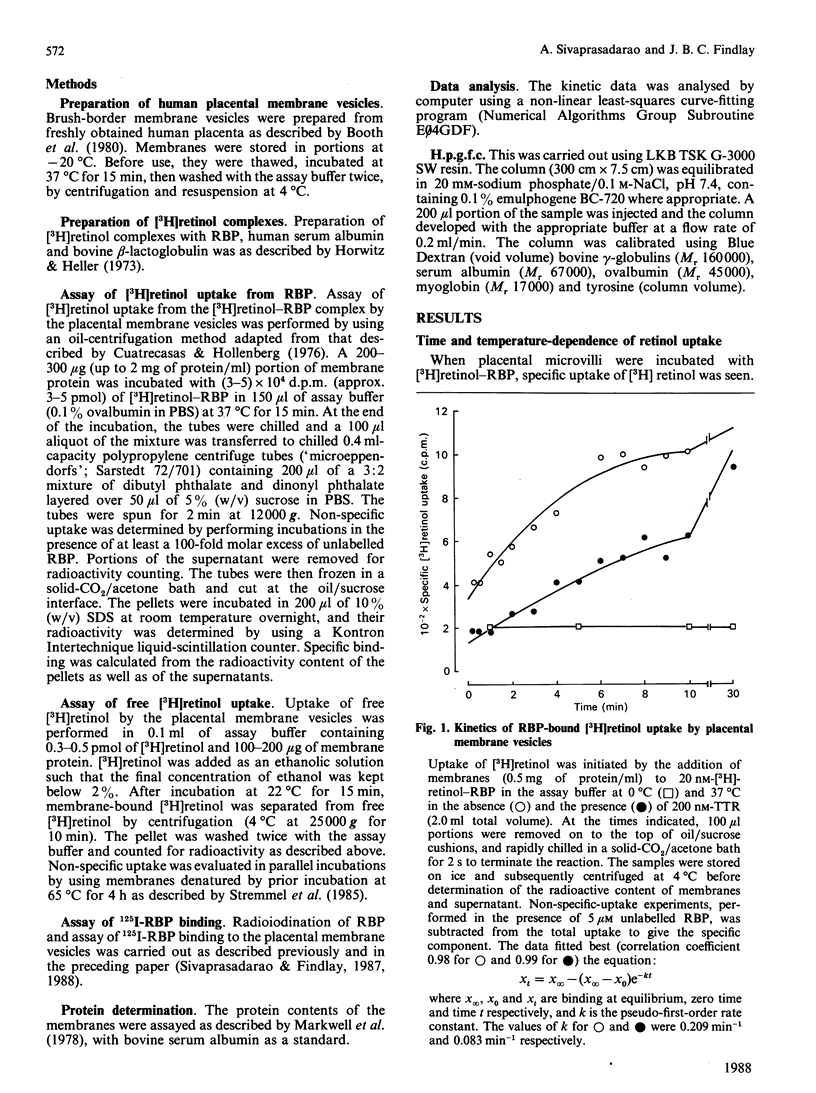
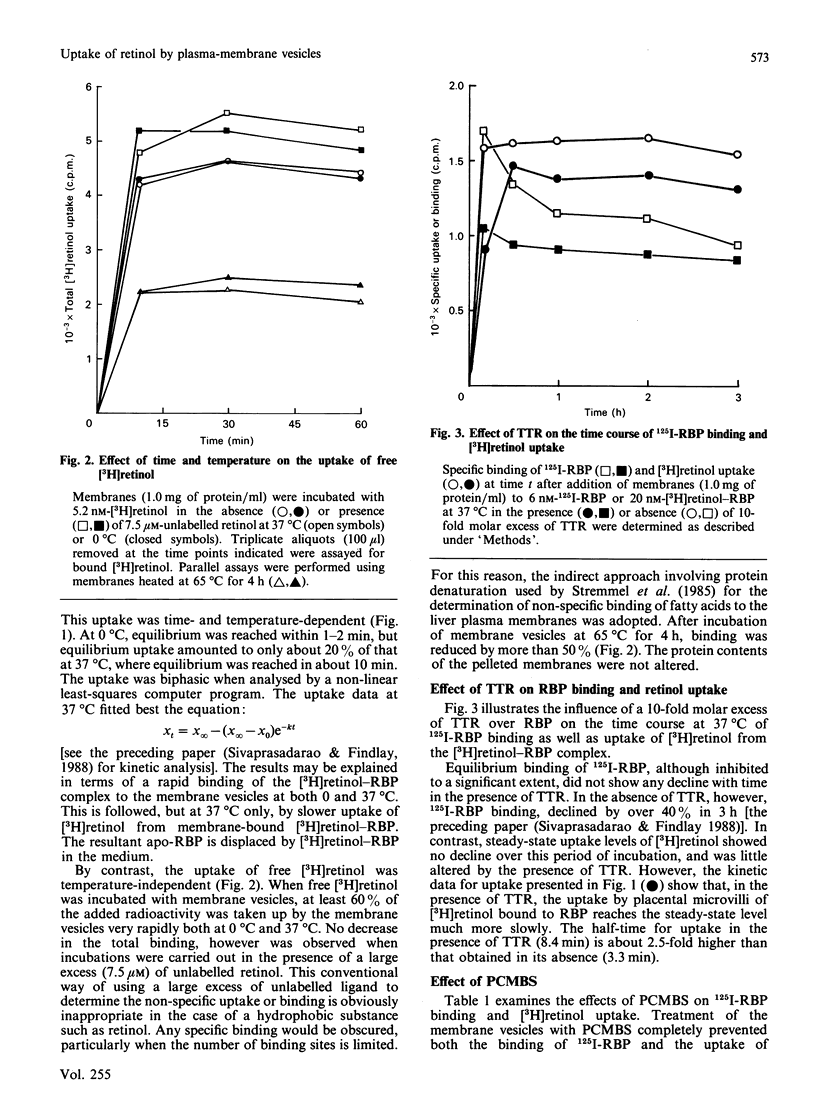
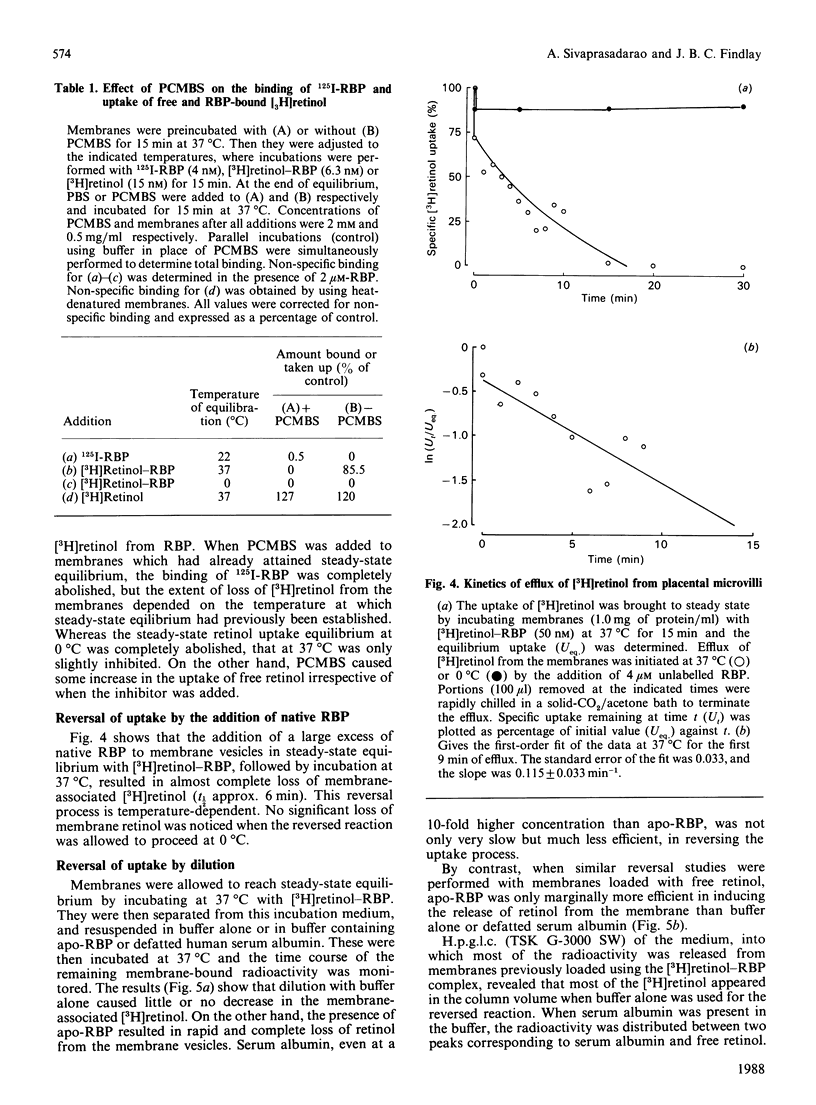
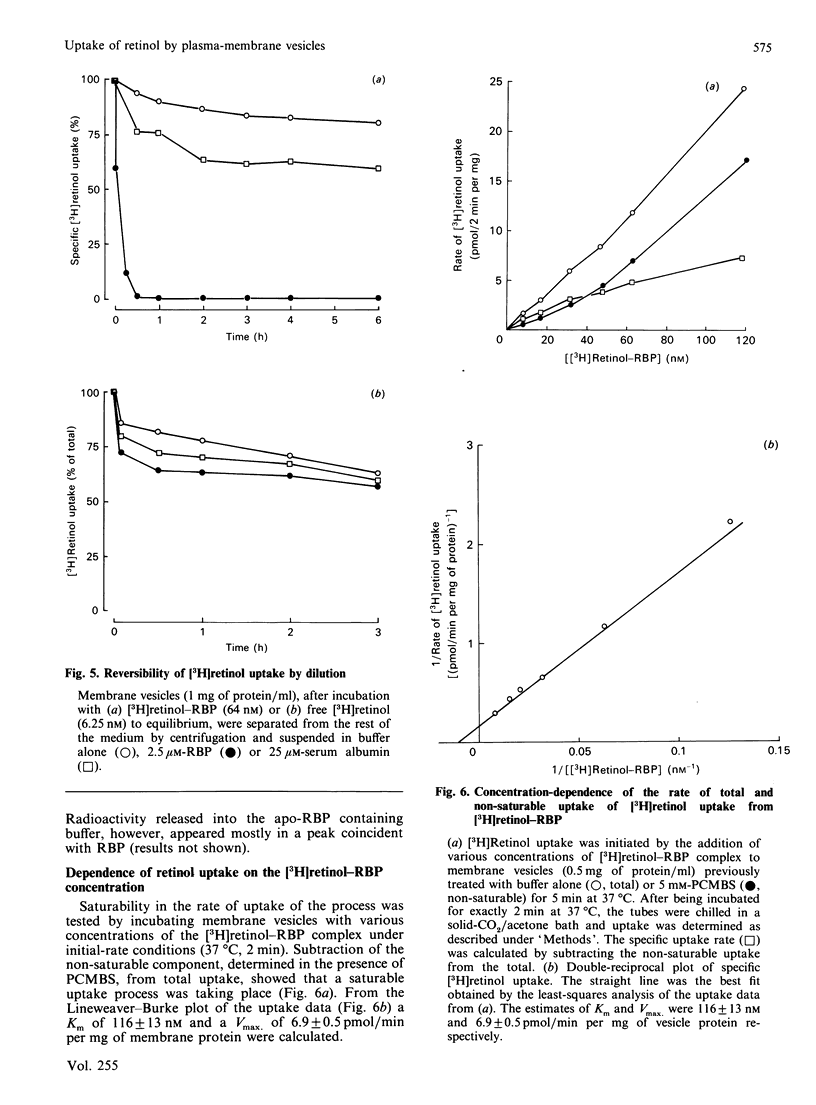
The mechanism of retinol uptake by human placental brush-border membrane vesicles was investigated using initial-velocity studies of [3H]retinol uptake from the [3H]retinol-RBP (retinol-binding protein) complex. The process was rapid and time- and temperature-dependent. The uptake was specifically reversed by the addition of native or apo-RBP, but not by serum albumin. By contrast, uptake of free [3H]retinol was temperature-independent, partially reversible and showed no requirement for a specific protein for reversibility. Treatment of membrane vesicles with p-chloromercuribenzenesulphonate (PCMBS), which inhibited 125I-RBP binding, also inhibited the uptake of retinol from RBP, but the uptake of free retinol was unaffected. Addition of PCMBS after the attainment of steady-state uptake equilibrium abolished the binding of RBP, but did not affect the retinol already taken up from RBP. The results suggest that binding of RBP to its specific receptor is obligatory for the subsequent delivery of retinol to the membrane. Since the studies were carried out on isolated membrane vesicles, endocytosis of RBP is most unlikely to be involved in the placental transport of retinol. A double-reciprocal plot of initial velocity versus [3H]retinol-RBP concentration gave an apparent Km of 116 +/- 13 nM. Transthyretin decreased the rate of uptake of [3H]retinol from RBP without substantially altering the steady-state uptake levels, suggesting that membranes take up retinol from uncomplexed RBP. High-pressure gel-filtration chromatography showed that [3H]retinol is largely transferred to a membrane component with an apparent molecular mass of 125 kDa.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomhoff R., Norum K. R., Berg T. Hepatic uptake of [3H]retinol bound to the serum retinol binding protein involves both parenchymal and perisinusoidal stellate cells. J Biol Chem. 1985 Nov 5;260(25):13571–13575. [PubMed] [Google Scholar]
- Booth A. G., Olaniyan R. O., Vanderpuye O. A. An improved method for the preparation of human placental syncytiotrophoblast microvilli. Placenta. 1980 Oct-Dec;1(4):327–336. doi: 10.1016/s0143-4004(80)80034-8. [DOI] [PubMed] [Google Scholar]
- Brecher P., Saouaf R., Sugarman J. M., Eisenberg D., LaRosa K. Fatty acid transfer between multilamellar liposomes and fatty acid-binding proteins. J Biol Chem. 1984 Nov 10;259(21):13395–13401. [PubMed] [Google Scholar]
- Chen C. C., Heller J. Uptake of retinol and retinoic acid from serum retinol-binding protein by retinal pigment epithelial cells. J Biol Chem. 1977 Aug 10;252(15):5216–5221. [PubMed] [Google Scholar]
- Cuatrecasas P., Hollenberg M. D. Membrane receptors and hormone action. Adv Protein Chem. 1976;30:251–451. doi: 10.1016/s0065-3233(08)60481-7. [DOI] [PubMed] [Google Scholar]
- Donoghue S., Richardson D. W., Sklan D., Kronfeld D. S. Placental transport of retinol in sheep. J Nutr. 1982 Nov;112(11):2197–2203. doi: 10.1093/jn/112.11.2197. [DOI] [PubMed] [Google Scholar]
- Fex G., Felding P. Factors affecting the concentration of free holo retinol-binding protein in human plasma. Eur J Clin Invest. 1984 Apr;14(2):146–149. doi: 10.1111/j.1365-2362.1984.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Green M. H., Uhl L., Green J. B. A multicompartmental model of vitamin A kinetics in rats with marginal liver vitamin A stores. J Lipid Res. 1985 Jul;26(7):806–818. [PubMed] [Google Scholar]
- Hamilton J. A., Cistola D. P. Transfer of oleic acid between albumin and phospholipid vesicles. Proc Natl Acad Sci U S A. 1986 Jan;83(1):82–86. doi: 10.1073/pnas.83.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J., Heller J. Interactions of all-trans, 9-, 11-, and 13-cis-retinal, all-trans-retinyl acetate, and retinoic acid with human retinol-binding protein and prealbumin. J Biol Chem. 1973 Sep 25;248(18):6317–6324. [PubMed] [Google Scholar]
- Hryb D. J., Khan M. S., Romas N. A., Rosner W. Specific binding of human corticosteroid-binding globulin to cell membranes. Proc Natl Acad Sci U S A. 1986 May;83(10):3253–3256. doi: 10.1073/pnas.83.10.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryb D. J., Khan M. S., Rosner W. Testosterone-estradiol-binding globulin binds to human prostatic cell membranes. Biochem Biophys Res Commun. 1985 Apr 16;128(1):432–440. doi: 10.1016/0006-291x(85)91697-3. [DOI] [PubMed] [Google Scholar]
- Ismadi S. D., Olson J. A. Dynamics of the fetal distribution and transfer of Vitamin A between rat fetuses and their mother. Int J Vitam Nutr Res. 1982;52(2):112–119. [PubMed] [Google Scholar]
- Lan N. C., Karin M., Nguyen T., Weisz A., Birnbaum M. J., Eberhardt N. L., Baxter J. D. Mechanisms of glucocorticoid hormone action. J Steroid Biochem. 1984 Jan;20(1):77–88. doi: 10.1016/0022-4731(84)90192-4. [DOI] [PubMed] [Google Scholar]
- Libby P. R., Bertram J. S. Lack of intracellular retinoid-binding proteins in a retinol-sensitive cell line. Carcinogenesis. 1982;3(5):481–484. doi: 10.1093/carcin/3.5.481. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D., Robson R. J., Dennis E. A. Solubilization of phospholipids by detergents. Structural and kinetic aspects. Biochim Biophys Acta. 1983 May 24;737(2):285–304. doi: 10.1016/0304-4157(83)90004-7. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McGuire B. W., Chytil F. Three-step purification of retinol-binding protein from rat serum. Biochim Biophys Acta. 1980 Feb 27;621(2):324–331. doi: 10.1016/0005-2795(80)90184-1. [DOI] [PubMed] [Google Scholar]
- Moore T. Vitamin A transfer from mother to offspring in mice and rats. Int J Vitam Nutr Res. 1971;41(3):301–306. [PubMed] [Google Scholar]
- Muto Y., Smith J. E., Milch P. O., Goodman D. S. Regulation of retinol-binding protein metabolism by vitamin A status in the rat. J Biol Chem. 1972 Apr 25;247(8):2542–2550. [PubMed] [Google Scholar]
- Oreffo R. O., Francis J. A., Triffitt J. T. Vitamin A effects on UMR 106 osteosarcoma cells are not mediated by specific cytosolic receptors. Biochem J. 1985 Dec 1;232(2):599–603. doi: 10.1042/bj2320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottonello S., Petrucco S., Maraini G. Vitamin A uptake from retinol-binding protein in a cell-free system from pigment epithelial cells of bovine retina. Retinol transfer from plasma retinol-binding protein to cytoplasmic retinol-binding protein with retinyl-ester formation as the intermediate step. J Biol Chem. 1987 Mar 25;262(9):3975–3981. [PubMed] [Google Scholar]
- Papiz M. Z., Sawyer L., Eliopoulos E. E., North A. C., Findlay J. B., Sivaprasadarao R., Jones T. A., Newcomer M. E., Kraulis P. J. The structure of beta-lactoglobulin and its similarity to plasma retinol-binding protein. 1986 Nov 27-Dec 3Nature. 324(6095):383–385. doi: 10.1038/324383a0. [DOI] [PubMed] [Google Scholar]
- Rask L., Peterson P. A. In vitro uptake of vitamin A from the retinol-binding plasma protein to mucosal epithelial cells from the monkey's small intestine. J Biol Chem. 1976 Oct 25;251(20):6360–6366. [PubMed] [Google Scholar]
- Rask L., Peterson P. A., Nilsson S. F. The subunit structure of human thyroxine-binding prealbumin. J Biol Chem. 1971 Oct 10;246(19):6087–6097. [PubMed] [Google Scholar]
- Sivaprasadarao A., Findlay J. B. The interaction of retinol-binding protein with its plasma-membrane receptor. Biochem J. 1988 Oct 15;255(2):561–569. [PMC free article] [PubMed] [Google Scholar]
- Soprano D. R., Soprano K. J., Goodman D. S. Retinol-binding protein and transthyretin mRNA levels in visceral yolk sac and liver during fetal development in the rat. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7330–7334. doi: 10.1073/pnas.83.19.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soprano D. R., Soprano K. J., Goodman D. S. Retinol-binding protein messenger RNA levels in the liver and in extrahepatic tissues of the rat. J Lipid Res. 1986 Feb;27(2):166–171. [PubMed] [Google Scholar]
- Strel'chyonok O. A., Avvakumov G. V., Survilo L. I. A recognition system for sex-hormone-binding protein-estradiol complex in human decidual endometrium plasma membranes. Biochim Biophys Acta. 1984 Dec 20;802(3):459–466. doi: 10.1016/0304-4165(84)90365-9. [DOI] [PubMed] [Google Scholar]
- Stremmel W., Strohmeyer G., Borchard F., Kochwa S., Berk P. D. Isolation and partial characterization of a fatty acid binding protein in rat liver plasma membranes. Proc Natl Acad Sci U S A. 1985 Jan;82(1):4–8. doi: 10.1073/pnas.82.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y. I., Smith J. E., Goodman D. S. Vitamin A and retinol-binding protein metabolism during fetal development in the rat. Am J Physiol. 1977 Oct;233(4):E263–E272. doi: 10.1152/ajpendo.1977.233.4.E263. [DOI] [PubMed] [Google Scholar]
- de Luca L. M., Bhat P. V., Sasak W., Adamo S. Biosynthesis of phosphoryl and glycosyl phosphoryl derivatives of vitamin A in biological membranes. Fed Proc. 1979 Oct;38(11):2535–2539. [PubMed] [Google Scholar]


