Abstract
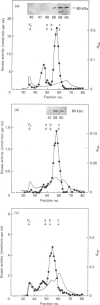
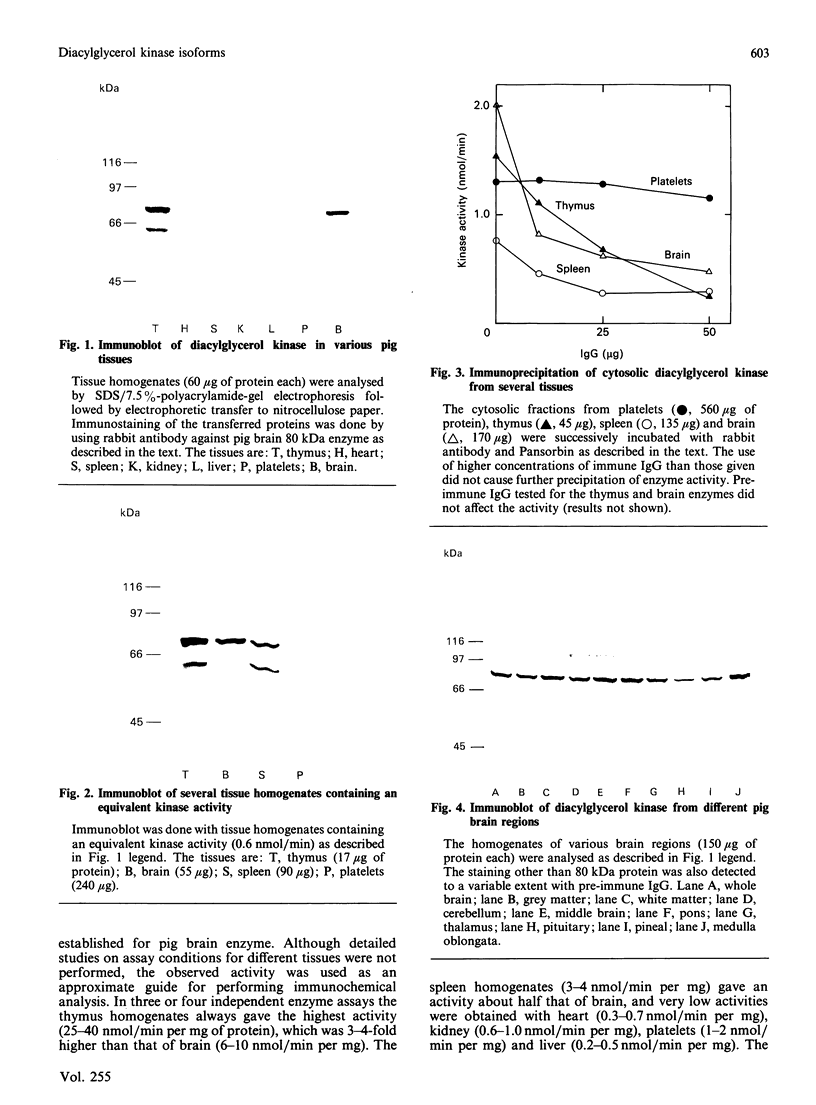
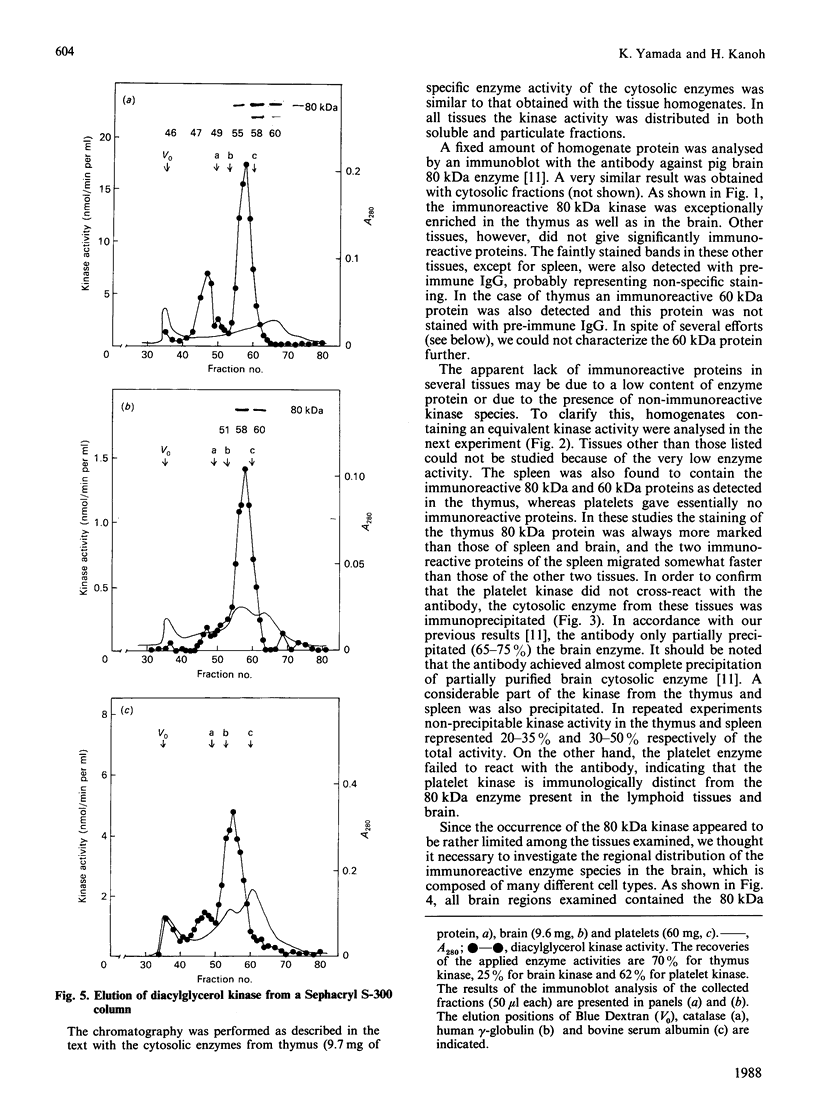
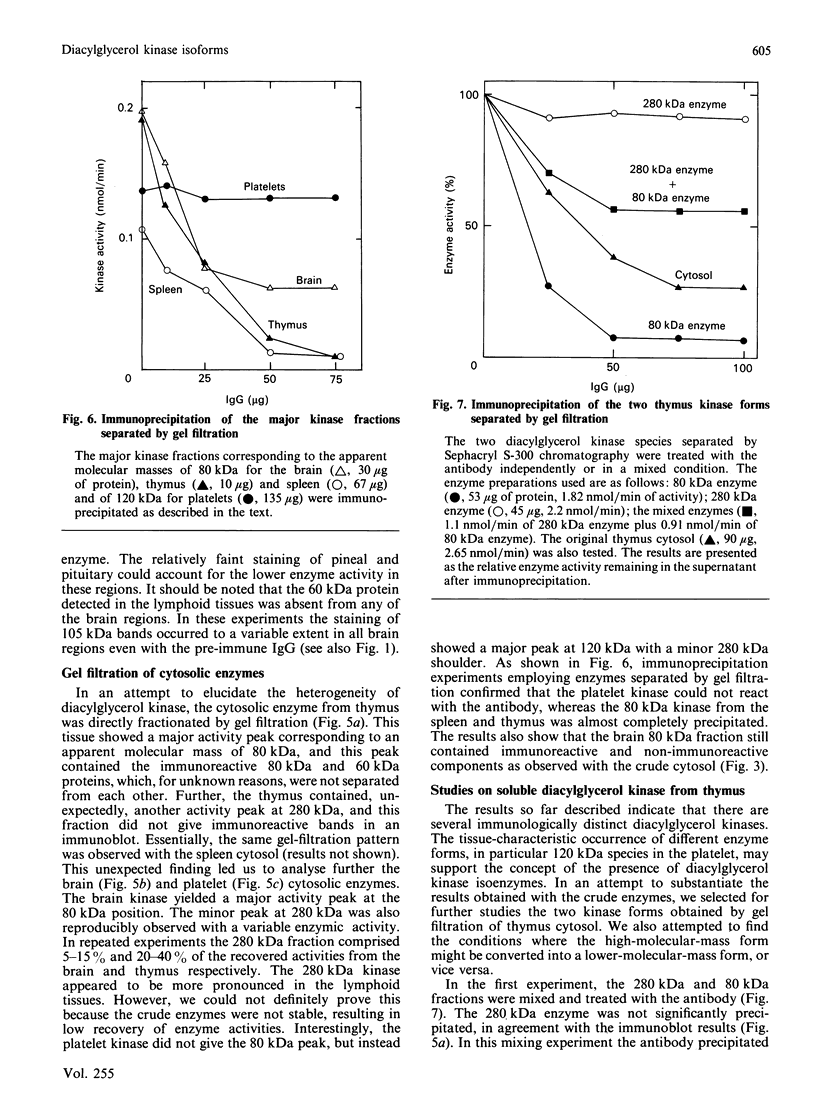
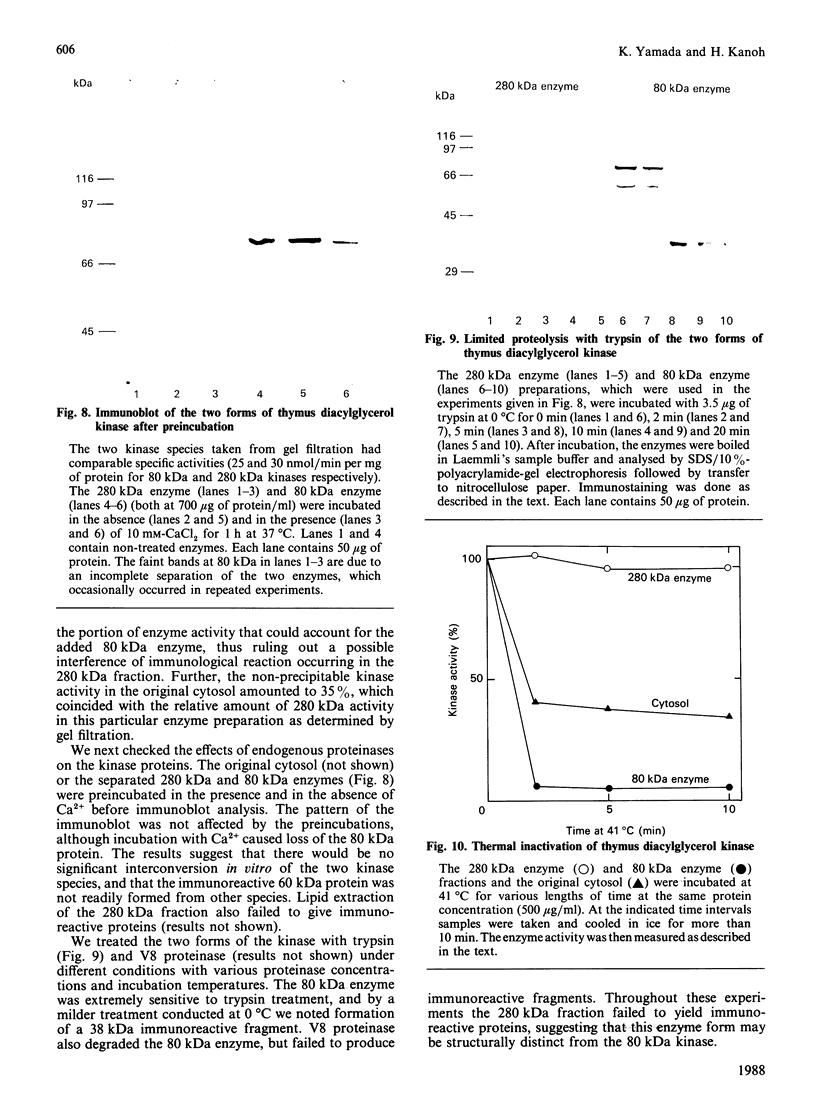
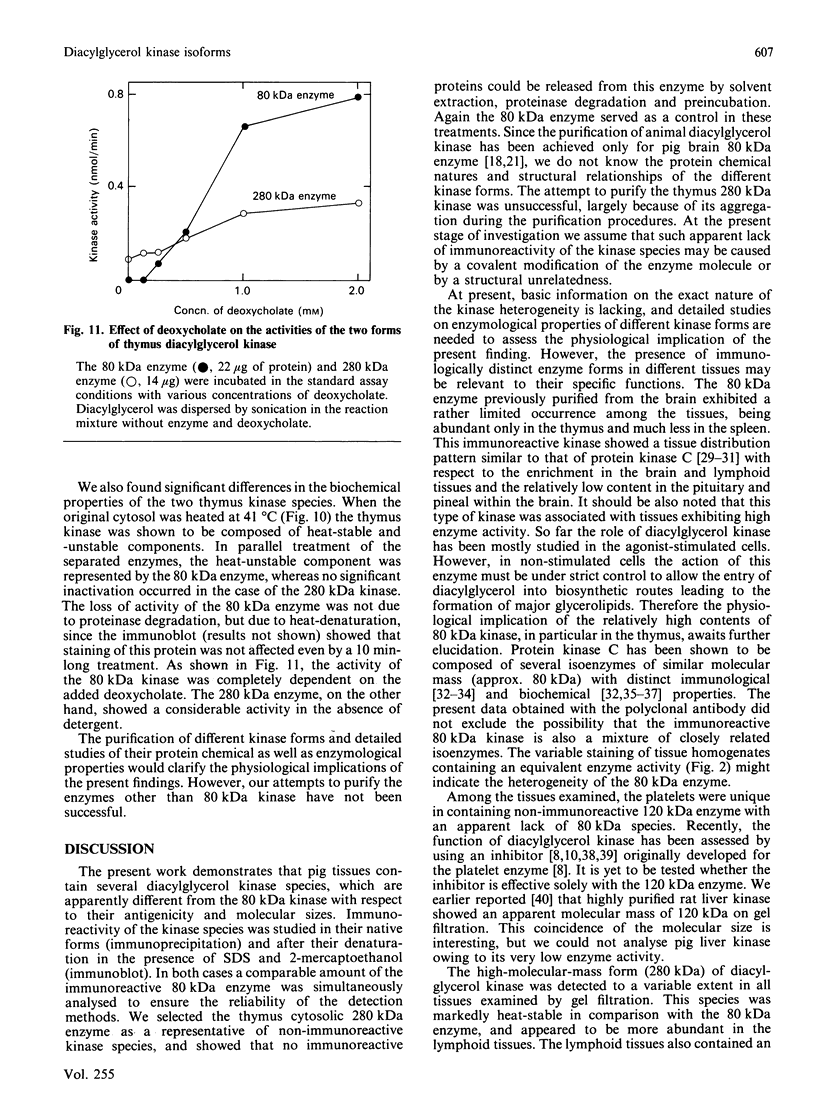
We surveyed diacylglycerol kinase in different pig tissues by using rabbit antibody immunospecific to the brain 80 kDa enzyme [Kanoh, Iwata, Ono & Suzuki (1986) J. Biol. Chem. 261, 5597-5602]. Among the other tissues examined, the immunoreactive 80 kDa enzyme was found only in the thymus and, to a much lesser extent, in the spleen, although this enzyme species was widely distributed in a variety of brain regions. Other tissues such as platelets, kidney, heart and liver contained little, if any, immunoreactive enzymes. Gel filtration of cytosolic enzymes from several tissues revealed the presence of three major activity peaks, apparently corresponding to 280, 120 and 80 kDa. Thymus and spleen contained the immunoreactive 80 kDa species together with non-immunoreactive 280 kDa enzyme. In the case of platelets, the kinase consisted almost exclusively of non-immunoreactive 120 kDa species with some 280 kDa enzyme. In an attempt to characterize the different kinase forms, the thymus enzyme was chosen for further studies because of its high activity. No immunoreactive proteins were detected in Western-blot analysis when the 280 kDa enzyme was solvent-extracted, proteinase-treated or preincubated in the presence of Ca2+. In comparison with the 80 kDa species, the 280 kDa enzyme was much more heat-stable and less dependent on deoxycholate in the assay mixture. Although the purification of different forms of the kinase is required to confirm the presence of isoenzymes, the results show that there exist several immunologically distinct diacylglycerol kinase species.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banno Y., Nozawa Y. Characterization of partially purified phospholipase C from human platelet membranes. Biochem J. 1987 Nov 15;248(1):95–101. doi: 10.1042/bj2480095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C. F., Crooke S. T. Purification and characterization of a phosphoinositide-specific phospholipase C from guinea pig uterus. Phosphorylation by protein kinase C in vivo. J Biol Chem. 1987 Oct 5;262(28):13789–13797. [PubMed] [Google Scholar]
- Besterman J. M., Pollenz R. S., Booker E. L., Jr, Cuatrecasas P. Diacylglycerol-induced translocation of diacylglycerol kinase: use of affinity-purified enzyme in a reconstitution system. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9378–9382. doi: 10.1073/pnas.83.24.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop W. R., Ganong B. R., Bell R. M. Attenuation of sn-1,2-diacylglycerol second messengers by diacylglycerol kinase. Inhibition by diacylglycerol analogs in vitro and in human platelets. J Biol Chem. 1986 May 25;261(15):6993–7000. [PubMed] [Google Scholar]
- Carter H. R., Smith A. D. Resolution of the phosphoinositide-specific phospholipase C isolated from porcine lymphocytes into multiple species. Partial purification of two isoenzymes. Biochem J. 1987 Jun 15;244(3):639–645. doi: 10.1042/bj2440639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cambronero J., Molski T. F., Becker E. L., Sha'afi R. I. The diacylglycerol kinase inhibitor R59022 potentiates superoxide production but not secretion induced by fMet-Leu-Phe: effects of leupeptin and the protein kinase C inhibitor H-7. Biochem Biophys Res Commun. 1987 Oct 14;148(1):38–46. doi: 10.1016/0006-291x(87)91073-4. [DOI] [PubMed] [Google Scholar]
- Hofmann S. L., Majerus P. W. Identification and properties of two distinct phosphatidylinositol-specific phospholipase C enzymes from sheep seminal vesicular glands. J Biol Chem. 1982 Jun 10;257(11):6461–6469. [PubMed] [Google Scholar]
- Hokin L. E. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Huang F. L. Immunochemical characterization of rat brain protein kinase C. J Biol Chem. 1986 Nov 5;261(31):14781–14787. [PubMed] [Google Scholar]
- Huang K. P., Nakabayashi H., Huang F. L. Isozymic forms of rat brain Ca2+-activated and phospholipid-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8535–8539. doi: 10.1073/pnas.83.22.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai A., Rebecchi M. J., Gershengorn M. C. Differential regulation by phosphatidylinositol 4,5-bisphosphate of pituitary plasma-membrane and cytosolic phosphoinositide kinases. Biochem J. 1986 Dec 1;240(2):341–348. doi: 10.1042/bj2400341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitoya J., Yamakawa A., Takenawa T. Translocation of diacylglycerol kinase in response to chemotactic peptide and phorbol ester in neutrophils. Biochem Biophys Res Commun. 1987 Apr 29;144(2):1025–1030. doi: 10.1016/s0006-291x(87)80066-9. [DOI] [PubMed] [Google Scholar]
- Jaken S., Kiley S. C. Purification and characterization of three types of protein kinase C from rabbit brain cytosol. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4418–4422. doi: 10.1073/pnas.84.13.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jergil B., Sundler R. Phosphorylation of phosphatidylinositol in rat liver Golgi. J Biol Chem. 1983 Jul 10;258(13):7968–7973. [PubMed] [Google Scholar]
- Kanoh H., Iwata T., Ono T., Suzuki T. Immunological characterization of sn-1,2-diacylglycerol and sn-2-monoacylglycerol kinase from pig brain. J Biol Chem. 1986 Apr 25;261(12):5597–5602. [PubMed] [Google Scholar]
- Kanoh H., Kondoh H., Ono T. Diacylglycerol kinase from pig brain. Purification and phospholipid dependencies. J Biol Chem. 1983 Feb 10;258(3):1767–1774. [PubMed] [Google Scholar]
- Kanoh H., Ohno K. Partial purification and properties of diacylglycerol kinase from rat liver cytosol. Arch Biochem Biophys. 1981 Jun;209(1):266–275. doi: 10.1016/0003-9861(81)90280-0. [DOI] [PubMed] [Google Scholar]
- Kanoh H., Ono T. Utilization of diacylglycerol in phospholipid bilayers by pig brain diacylglycerol kinase and Rhizopus arrhizus lipase. J Biol Chem. 1984 Sep 25;259(18):11197–11202. [PubMed] [Google Scholar]
- Kuo J. F., Andersson R. G., Wise B. C., Mackerlova L., Salomonsson I., Brackett N. L., Katoh N., Shoji M., Wrenn R. W. Calcium-dependent protein kinase: widespread occurrence in various tissues and phyla of the animal kingdom and comparison of effects of phospholipid, calmodulin, and trifluoperazine. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7039–7043. doi: 10.1073/pnas.77.12.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Billah M. M., Cuatrecasas P. Lysophosphatidic acid potentiates the thrombin-induced production of arachidonate metabolites in platelets. J Biol Chem. 1981 Dec 10;256(23):11984–11987. [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D., Basbaum A. I., Koshland D. E., Jr Distinct cellular and regional localization of immunoreactive protein kinase C in rat brain. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4660–4664. doi: 10.1073/pnas.84.13.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muid R. E., Penfield A., Dale M. M. The diacylglycerol kinase inhibitor, R59022, enhances the superoxide generation from human neutrophils induced by stimulation of fMet-Leu-Phe, IgG and C3b receptors. Biochem Biophys Res Commun. 1987 Mar 13;143(2):630–637. doi: 10.1016/0006-291x(87)91400-8. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Phosphatidic acid may stimulate membrane receptors mediating adenylate cyclase inhibition and phospholipid breakdown in 3T3 fibroblasts. J Biol Chem. 1987 Apr 25;262(12):5522–5529. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nunn D. L., Watson S. P. A diacylglycerol kinase inhibitor, R59022, potentiates secretion by and aggregation of thrombin-stimulated human platelets. Biochem J. 1987 May 1;243(3):809–813. doi: 10.1042/bj2430809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y., Takai Y., Kawahara Y., Kimura S., Nishizuka Y. A new possible regulatory system for protein phosphorylation in human peripheral lymphocytes. I. Characterization of a calcium-activated, phospholipid-dependent protein kinase. J Immunol. 1981 Oct;127(4):1369–1374. [PubMed] [Google Scholar]
- Ohsako S., Deguchi T. Stimulation of phosphatidic acid of calcium influx and cyclic GMP synthesis in neuroblastoma cells. J Biol Chem. 1981 Nov 10;256(21):10945–10948. [PubMed] [Google Scholar]
- Putney J. W., Jr, Weiss S. J., Van De Walle C. M., Haddas R. A. Is phosphatidic acid a calcium ionophore under neurohumoral control? Nature. 1980 Mar 27;284(5754):345–347. doi: 10.1038/284345a0. [DOI] [PubMed] [Google Scholar]
- Salmon D. M., Honeyman T. W. Proposed mechanism of cholinergic action in smooth muscle. Nature. 1980 Mar 27;284(5754):344–345. doi: 10.1038/284344a0. [DOI] [PubMed] [Google Scholar]
- Saltiel A. R., Fox J. A., Sherline P., Sahyoun N., Cuatrecasas P. Purification of phosphatidylinositol kinase from bovine brain myelin. Biochem J. 1987 Feb 1;241(3):759–763. doi: 10.1042/bj2410759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi K., Tsukuda M., Ogita K., Kikkawa U., Nishizuka Y. Three distinct forms of rat brain protein kinase C: differential response to unsaturated fatty acids. Biochem Biophys Res Commun. 1987 Jun 15;145(2):797–802. doi: 10.1016/0006-291x(87)91035-7. [DOI] [PubMed] [Google Scholar]
- Siess W., Siegel F. L., Lapetina E. G. Arachidonic acid stimulates the formation of 1,2-diacylglycerol and phosphatidic acid in human platelets. Degree of phospholipase C activation correlates with protein phosphorylation, platelet shape change, serotonin release, and aggregation. J Biol Chem. 1983 Sep 25;258(18):11236–11242. [PubMed] [Google Scholar]
- Sugimoto Y., Erikson R. L. Phosphatidylinositol kinase activities in normal and Rous sarcoma virus-transformed cells. Mol Cell Biol. 1985 Nov;5(11):3194–3198. doi: 10.1128/mcb.5.11.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenawa T., Nagai Y. Purification of phosphatidylinositol-specific phospholipase C from rat liver. J Biol Chem. 1981 Jul 10;256(13):6769–6775. [PubMed] [Google Scholar]
- Tokumura A., Fukuzawa K., Isobe J., Tsukatani H. Lysophosphatidic acid-induced aggregation of human and feline platelets: structure-activity relationship. Biochem Biophys Res Commun. 1981 Mar 31;99(2):391–398. doi: 10.1016/0006-291x(81)91758-7. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R., Hunter T. Immunological evidence for two physiological forms of protein kinase C. Mol Cell Biol. 1987 Jan;7(1):85–96. doi: 10.1128/mcb.7.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett J. R., Hunter T. Isolation and characterization of two distinct forms of protein kinase C. J Biol Chem. 1987 Apr 5;262(10):4836–4843. [PubMed] [Google Scholar]
- de Chaffoy de Courcelles D. C., Roevens P., Van Belle H. R 59 022, a diacylglycerol kinase inhibitor. Its effect on diacylglycerol and thrombin-induced C kinase activation in the intact platelet. J Biol Chem. 1985 Dec 15;260(29):15762–15770. [PubMed] [Google Scholar]