Abstract
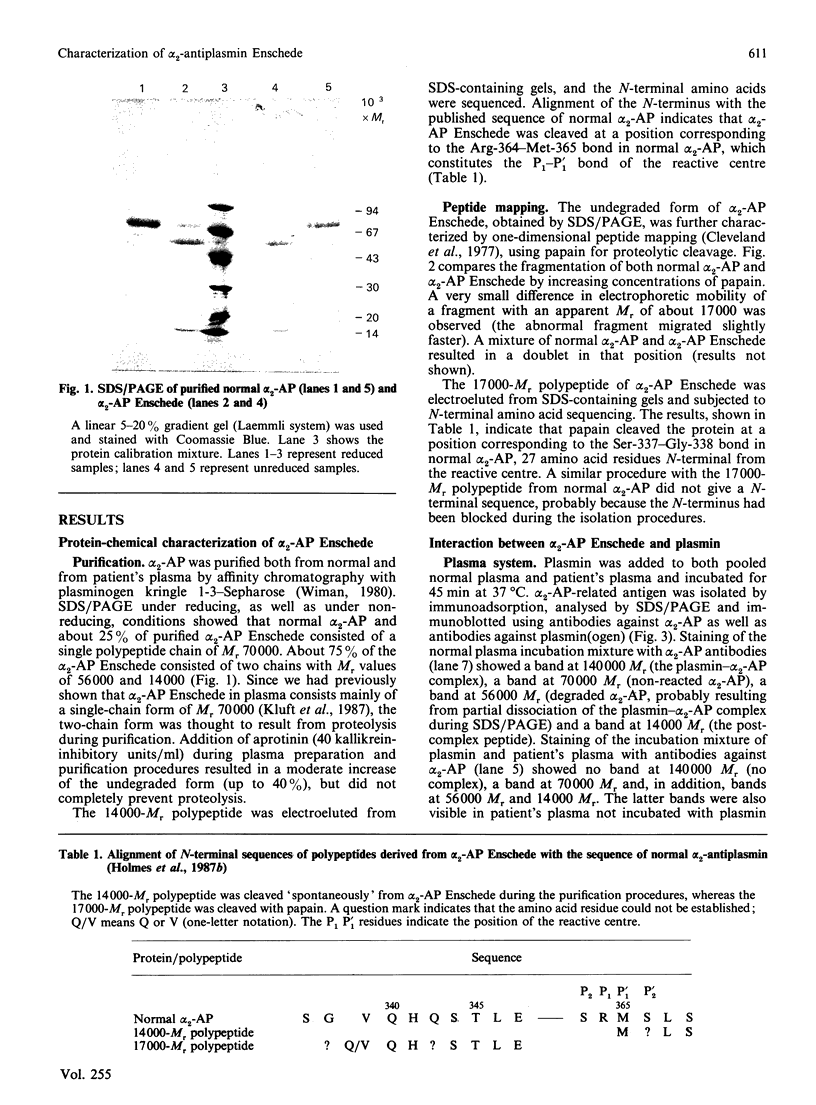
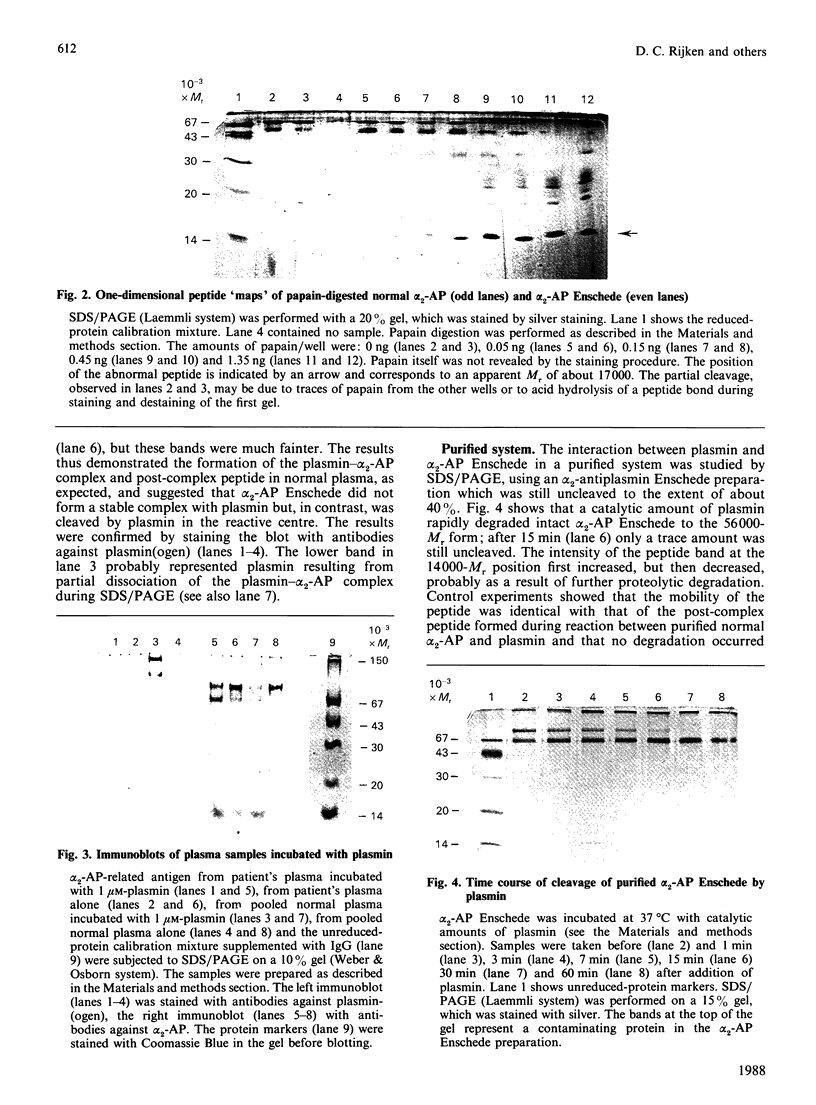
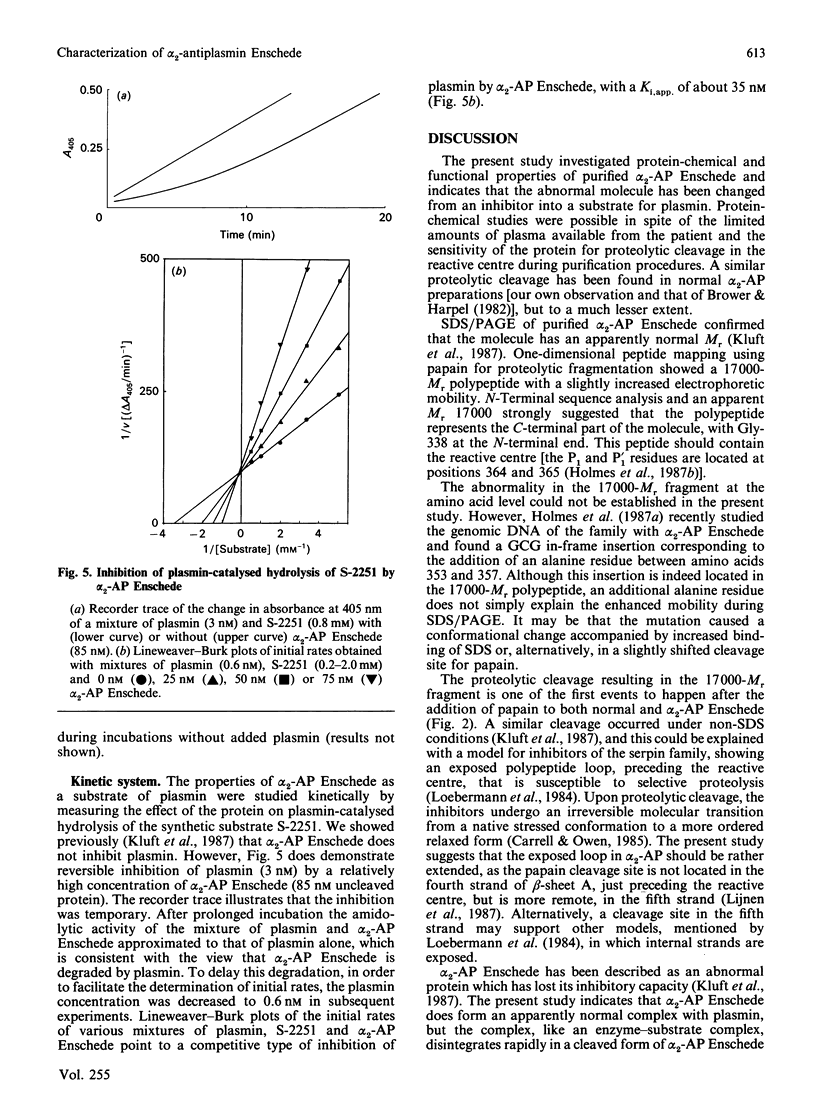
alpha 2-Antiplasmin Enschede is a variant of alpha 2-antiplasmin which has lost its ability to inhibit plasmin irreversibly and which is associated with a haemorrhagic disorder [Kluft et al. (1987) J. Clin. Invest. 80, 1391-1400]. The abnormal protein was purified from the plasma of a homozygous patient and subjected to one-dimensional peptide mapping using papain for digestion. A slightly abnormally migrating polypeptide (Mr 17,000) was found which represented the C-terminal part of the molecule (the N-terminus of the polypeptide corresponded to Gly-338 in normal alpha 2-antiplasmin) and which contained the reactive centre. The interaction of plasmin with alpha 2-antiplasmin Enschede was studied by adding plasmin to plasma of the homozygous patient. SDS/polyacrylamide-gel electrophoresis and immunoblotting showed that no complex persisted, but that the abnormal alpha 2-antiplasmin was cleaved into two fragments of Mr 56,000 and 14,000 respectively. The latter fragment co-migrated with the post-complex peptide, which is cleaved from normal alpha 2-antiplasmin during complex-formation with plasmin. In a purified system, catalytic amounts of plasmin rapidly cleaved alpha 2-antiplasmin Enschede into the aforementioned fragments. In kinetic studies alpha 2-antiplasmin Enschede reversibly and temporarily inhibited the plasmin-catalysed hydrolysis of D-valyl-L-leucyl-L-lysine p-nitroanilide ('S-2251') as a competitive inhibitor (Ki,app. 35 nM). It was concluded that alpha 2-antiplasmin Enschede apparently forms a normal complex with plasmin. The complex is, however, not stable, but disintegrates rapidly to a cleaved form of alpha 2-antiplasmin Enschede and active plasmin. The abnormal protein thus behaves like a substrate, instead of an inhibitor, of plasmin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amons R., Schrier P. I. Removal of sodium dodecyl sulfate from proteins and peptides by gel filtration. Anal Biochem. 1981 Sep 15;116(2):439–443. doi: 10.1016/0003-2697(81)90385-7. [DOI] [PubMed] [Google Scholar]
- Aoki N., Saito H., Kamiya T., Koie K., Sakata Y., Kobakura M. Congenital deficiency of alpha 2-plasmin inhibitor associated with severe hemorrhagic tendency. J Clin Invest. 1979 May;63(5):877–884. doi: 10.1172/JCI109387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Brennan S. O., Carrell R. W. alpha 1-Antitrypsin Christchurch, 363 Glu----Lys: mutation at the P'5 position does not affect inhibitory activity. Biochim Biophys Acta. 1986 Sep 5;873(1):13–19. doi: 10.1016/0167-4838(86)90183-4. [DOI] [PubMed] [Google Scholar]
- Brower M. S., Harpel P. C. Proteolytic cleavage and inactivation of alpha 2-plasmin inhibitor and C1 inactivator by human polymorphonuclear leukocyte elastase. J Biol Chem. 1982 Aug 25;257(16):9849–9854. [PubMed] [Google Scholar]
- Carrell R. W., Owen M. C. Plakalbumin, alpha 1-antitrypsin, antithrombin and the mechanism of inflammatory thrombosis. Nature. 1985 Oct 24;317(6039):730–732. doi: 10.1038/317730a0. [DOI] [PubMed] [Google Scholar]
- Chang J. Y., Tran T. H. Antithrombin III Basel. Identification of a Pro-Leu substitution in a hereditary abnormal antithrombin with impaired heparin cofactor activity. J Biol Chem. 1986 Jan 25;261(3):1174–1176. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collen D. Identification and some properties of a new fast-reacting plasmin inhibitor in human plasma. Eur J Biochem. 1976 Oct 1;69(1):209–216. doi: 10.1111/j.1432-1033.1976.tb10875.x. [DOI] [PubMed] [Google Scholar]
- Holmes W. E., Lijnen H. R., Nelles L., Kluft C., Nieuwenhuis H. K., Rijken D. C., Collen D. Alpha 2-antiplasmin Enschede: alanine insertion and abolition of plasmin inhibitory activity. Science. 1987 Oct 9;238(4824):209–211. doi: 10.1126/science.2958938. [DOI] [PubMed] [Google Scholar]
- Holmes W. E., Nelles L., Lijnen H. R., Collen D. Primary structure of human alpha 2-antiplasmin, a serine protease inhibitor (serpin). J Biol Chem. 1987 Feb 5;262(4):1659–1664. [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Kluft C., Nieuwenhuis H. K., Rijken D. C., Groeneveld E., Wijngaards G., van Berkel W., Dooijewaard G., Sixma J. J. alpha 2-Antiplasmin Enschede: dysfunctional alpha 2-antiplasmin molecule associated with an autosomal recessive hemorrhagic disorder. J Clin Invest. 1987 Nov;80(5):1391–1400. doi: 10.1172/JCI113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluft C., Traas D. W., Jie A. F., Hoegee-de Nobel E. The suitability of various plasmin preparations for the functional assay of alpha 2-antiplasmin in plasma. Thromb Haemost. 1982 Dec 27;48(3):320–324. [PubMed] [Google Scholar]
- Kluft C., Vellenga E., Brommer E. J., Wijngaards G. A familial hemorrhagic diathesis in a Dutch family: an inherited deficiency of alpha 2-antiplasmin. Blood. 1982 Jun;59(6):1169–1180. [PubMed] [Google Scholar]
- Koide T., Odani S., Takahashi K., Ono T., Sakuragawa N. Antithrombin III Toyama: replacement of arginine-47 by cysteine in hereditary abnormal antithrombin III that lacks heparin-binding ability. Proc Natl Acad Sci U S A. 1984 Jan;81(2):289–293. doi: 10.1073/pnas.81.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lijnen H. R., Holmes W. E., van Hoef B., Wiman B., Rodriguez H., Collen D. Amino-acid sequence of human alpha 2-antiplasmin. Eur J Biochem. 1987 Aug 3;166(3):565–574. doi: 10.1111/j.1432-1033.1987.tb13551.x. [DOI] [PubMed] [Google Scholar]
- Loebermann H., Tokuoka R., Deisenhofer J., Huber R. Human alpha 1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol. 1984 Aug 15;177(3):531–557. [PubMed] [Google Scholar]
- Miles L. A., Plow E. F., Donnelly K. J., Hougie C., Griffin J. H. A bleeding disorder due to deficiency of alpha 2-antiplasmin. Blood. 1982 Jun;59(6):1246–1251. [PubMed] [Google Scholar]
- Moroi M., Aoki N. Isolation and characterization of alpha2-plasmin inhibitor from human plasma. A novel proteinase inhibitor which inhibits activator-induced clot lysis. J Biol Chem. 1976 Oct 10;251(19):5956–5965. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Müllertz S., Clemmensen I. The primary inhibitor of plasmin in human plasma. Biochem J. 1976 Dec 1;159(3):545–553. doi: 10.1042/bj1590545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M. C., Borg J. Y., Soria C., Soria J., Caen J., Carrell R. W. Heparin binding defect in a new antithrombin III variant: Rouen, 47 Arg to His. Blood. 1987 May;69(5):1275–1279. [PubMed] [Google Scholar]
- Owen M. C., Brennan S. O., Lewis J. H., Carrell R. W. Mutation of antitrypsin to antithrombin. alpha 1-antitrypsin Pittsburgh (358 Met leads to Arg), a fatal bleeding disorder. N Engl J Med. 1983 Sep 22;309(12):694–698. doi: 10.1056/NEJM198309223091203. [DOI] [PubMed] [Google Scholar]
- Shieh B. H., Travis J. The reactive site of human alpha 2-antiplasmin. J Biol Chem. 1987 May 5;262(13):6055–6059. [PubMed] [Google Scholar]
- Stephens A. W., Thalley B. S., Hirs C. H. Antithrombin-III Denver, a reactive site variant. J Biol Chem. 1987 Jan 25;262(3):1044–1048. [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wiman B. Affinity-chromatographic purification of human alpha 2-antiplasmin. Biochem J. 1980 Oct 1;191(1):229–232. doi: 10.1042/bj1910229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiman B., Collen D. On the mechanism of the reaction between human alpha 2-antiplasmin and plasmin. J Biol Chem. 1979 Sep 25;254(18):9291–9297. [PubMed] [Google Scholar]