ABSTRACT
Cemiplimab has demonstrated relevant clinical activity in cutaneous squamous cell carcinoma (cSCC) but mechanisms of primary and acquired resistance to immunotherapy are still unknown. We collected clinical data from locally advanced and/or metastatic cSSC patients treated with cemiplimab in two Italian University centers. In addition, gene expression analysis by using Nanostring Technologies platform to evaluate 770 cancer- and immune-related genes on 20 tumor tissue samples (9 responders and 11 non-responders to cemiplimab) was performed. We enrolled 81 patients with a median age of 82 years. After 16.4 months of median follow-up, 12- and 24-months PFS were 53% and 42%, respectively; while 12- and 24-months OS were 71% and 61%, respectively. Treatment was well tolerated. Overall response rate (ORR) was 58%, with a disease control rate (DCR) of 77.8%. The difference between genes expressed in responder versus non-responder patient samples was substantial, particularly for genes involved in immune system regulation. Cemiplimab-resistant tumors were associated with over-expression of CCL-20 and CXCL-8. Cemiplimab confirmed efficacy and safety data in real-life cSCC patients. Overexpression of CCL-20 and CXCL-8 could represent biomarkers of lack of response to immunotherapy.
KEYWORDS: Cemiplimab, cSCC, cutaneous squamous cell carcinoma, gene expression profiling, immunotherapy, nanostring, real-world data
Background
Cutaneous squamous cell carcinoma (cSCC) is the second most common type of skin cancer, which is typically observed in elderly patients with a clinical history of chronic sun exposure.1 Its incidence has increased in recent decades and, if early treated with surgery, the 5-year cure rate is over 90%.1 In a minority of cases, due to patient neglect or to the aggressiveness of the disease, it is diagnosed in locally advanced (lacSCC) or metastatic (mcSCC) stages. Due to the peculiar evolution of this tumor and its clinical presentation, staging is quite complex. In fact, the definition of lacSCC is not well defined, by including tumors that cannot be cured with surgery, radiation therapy (RT), or by a combination of the two treatments.1 Until a few years ago, for these subgroups of patients, there were limited systemic therapies with controversial efficacy. In fact, chemotherapy (platinum-based), or epidermal growth factor receptor (EGFR) inhibitors (cetuximab) have shown limited benefit at the cost of relevant toxicities. These data derive from small and mostly retrospective clinical trials.2 However, considering the high rate of somatic mutations (caused by UV radiation),3 since 2015 immunotherapy has been tested in cSCCin particular, by using programmed cell death-1 (PD-1) inhibitors. Cemiplimab, a fully human IgG4 monoclonal antibody, which is directed against PD-1, was the first drug approved by the Food and Drug administration (FDA) for mcSCC and lacSCC, in September 2018. Indeed, cemiplimab demonstrated durable clinical responses with prolonged survival in both phase I4 and phase II5 trials. Nonetheless, there is paucity of real-world data in this context. Furthermore, despite a high overall response rate (ORR), there are some patients with resistance to cemiplimab and no biomarkers exist to identify them. For these reasons, the aim of the present work was to identify potential biomarkers of response or of resistance to cemiplimab therapy in a real-world population of lacSCC and mcSCC patients treated with cemiplimab in two Academic Italian institution.
Methods
Study oversight
We retrospectively investigated clinical data from patients with lacSCC and mcSCC treated with cemiplimab from May 2020 to December 2022 at the Oncology Divisions of University of Campania “Luigi Vanvitelli” and of University of Naples “Federico II”, Italy. Patients with the following characteristics were included in the analysis: age ≥18 years; histopathological diagnosis of lacSCC not amenable for curative surgery or radiotherapy or mcSCC; at least one cycle of cemiplimab as first-line systemic therapy. Patients received cemiplimab intravenously over 30 min at a flat dose of 350 mg every 21 days until disease progression or unacceptable toxicity. The assessments of tumor response were performed every two cycles by digital medical photographs of the superficial lesions and every 3 months by Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) scan.
All patient information was recorded in an internal computer database. The retrospective study protocol was approved by the institutional review board at the main study site (University of Campania “Luigi Vanvitelli”). The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients signed a written informed consent and agreed with the research use of their anonymized data. Data regarding adverse events were collected and graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 5.0. Data cutoff for analysis was 31/12/2022. Survival curves were generated based on the Kaplan–Meier method. Statistical significance of survival curves was calculated using the Log-rank test. SPSS package (version 23, IBM) was used to generate survival curves and to calculate statistics throughout the entire manuscript. A p value of less than 0.05 was considered statistically significant.
Nanostring nCounter analysis
RNA from paraffin-embedded (FPPE) tumor biopsies of 20 tumor samples and 3 healthy samples was extracted using Maxwell® RSC RNA FFPE Kit (Promega) by using Maxwell RSC Instrument of Promega Corporation according to manufacturers’ standard protocol. Total RNA quantification and quality analysis was performed using TapeStation.
Gene expression and gene set analysis on pre-treated formalin-fixed tissues were performed using Nanostring Technologies platform (nCounter Analysis System). nCounter Nanostring Analysis using PanCancer IO 360 model Panel measured 770 cancer- and immune-related genes from RNA of selected patients according to the manufacturers’ standard protocol. Data were normalized according to positive and negative controls, and then mRNA counts were log transformed for following analysis. Gene set enrichment analysis (GSEA) was performed on normalized data obtained by nCounter system to pinpoint specific gene signatures in pre-cemiplimab samples associated with pathological complete response or residual disease after therapy.
Results
Patients characteristics
We enrolled 81 patients (Table 1) with lacSCC or mcSCC, of which 71.6% were male, with a median age at diagnosis of 82 years and a majority of them with performance status (PS) equal or greater than 1. Most of primary tumors were localized on head or neck (76.5%). Thirty-three patients (40.7%) had neither surgery nor radiotherapy before starting cemiplimab, while 45 patients (55.6%) had already received at least one surgery and 28 patients (34.6%) had radiotherapy treatment prior immunotherapy (at least 6 months before). None of the patients received systemic treatment prior to cemiplimab. During the treatment, nine patients received concomitant RT on primary tumor in order to maximize the response or to manage a local disease progression and continue cemiplimab beyond progression.
Table 1.
Baseline patient characteristics.
| Characteristics | Patient number, N=81 (%) |
|---|---|
| Sex | |
| Male | 58 (71.6) |
| Female | 23 (28.4) |
| Median age at diagnosis | 82y (48–97) |
| Basal Performance Status (ECOG) | |
| 0 | 14 (17.3) |
| 1 | 54 (66.7) |
| 2 | 13 (16) |
| Primary site | |
| Head/neck | 62 (76.5) |
| Trunk | 4 (4.9) |
| Upper limbs | 4 (4.9) |
| Lower limbs | 11 (13.6) |
| N. of previous surgery | |
| No previous surgery | 36 (44.4) |
| 1 | 22 (27.2) |
| ≥2 | 23 (28.4) |
| Previous RT | |
| Yes | 28 (34.6) |
| No | 53 (65.4) |
| T status | |
| T1–3 | 31 (38.3) |
| T4 | 50 (61.7) |
| N status | |
| N0 | 51 (63) |
| N+ | 30 (37) |
| M status | |
| M0 | 72 (88.9) |
| M1 | 9 (11.1) |
Efficacy
At data cutoff, 41 patients (50.6%) had a relapse or died, and 33 patients were on treatment with cemiplimab. After 16.4 months of median follow-up, median progression free survival (PFS) was 12.6 months (Figure 1a). 12- and 24-months PFS were 53% and 42%, respectively. There was a non-significant difference in terms of PFS between patients, that had ≥2 radical surgeries and those who had 0–1 (HR: 0.58 Confidence Interval (CI) 95% 0.31–1.07 p = 0.084) (Supplementary Figure S1a). No difference was observed in PFS between patients that had received RT on the primary tumor prior to cemiplimab administration and those patients that had not obtained RT (HR: 0.67 CI 95% 0.34–1.3 p = 0.23) (Supplementary Figure S1b).
Figure 1.
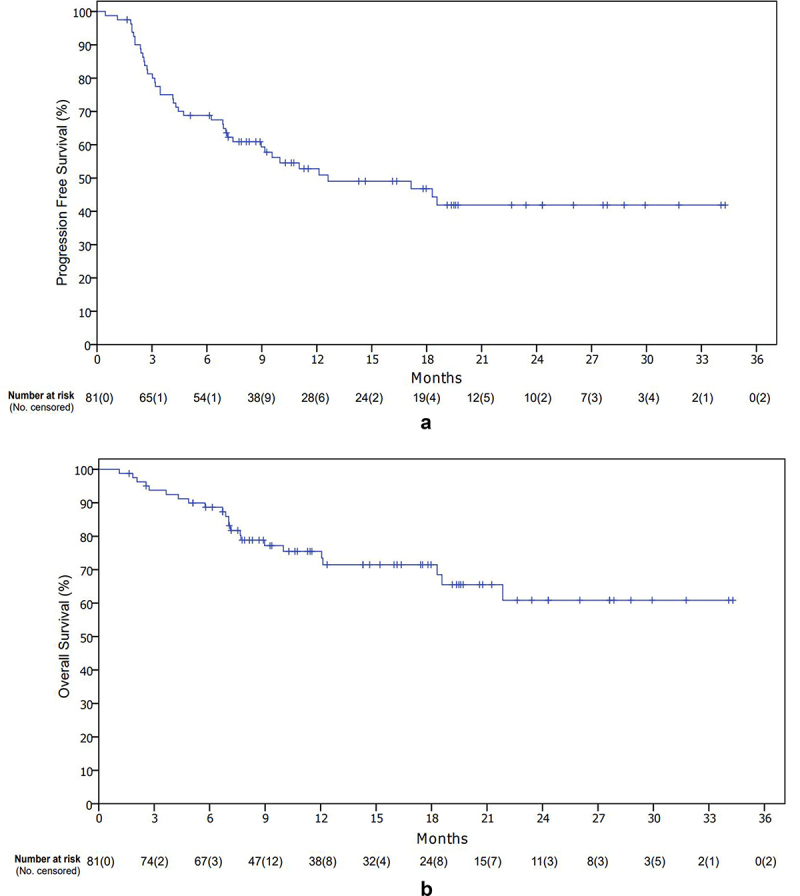
Kaplan–Meier curves: progression free survival (a) and overall survival (b).
Median OS was not reached (Figure 1b). 12- and 24-months OS were 71% and 61%, respectively. A statistically significant difference in the stratified subgroups for previous surgery was observed (Yes vs No HR: 0.38 CI 95% 0.16–0.90 p = 0.028) (Supplementary Figure S2a). Similarly, for PFS, no difference in the stratified group for previous radiotherapy was observed (Yes vs No HR: 1.21 CI 95% 0.52–2.70 p = 0.65) (Supplementary Figure S2b). Overall response rate (ORR) was 58%, of which 14 complete response (CR) (17.3%) (Table 2). Median duration of response (DOR) was not reached. ORR for patients with head/neck tumors was higher compared to other primary tumor sites (62.9% vs 42.1%). Median time to response was 3 months. Six patients were not evaluable for response. Disease control rate (DCR) was 77.8%. Bar graph for patients experiencing an objective response is shown in Figure 2. At progression of disease, most of the patients were candidates for best supportive care (BSC), while 9 patients received RT plus cemiplimab beyond progression, 2 received platinum-based chemotherapy, 1 received electrochemotherapy and 2 received palliative surgery or radiotherapy alone.
Table 2.
Table for response according to RECIST version 1.1. ORR = overall response rate; PR = partial response; CR = complete response; SD = stable disease; DCR = disease control rate; PD = progression disease; NA = data not available.
| N (%) | |
|---|---|
| ORR | 47 (58) |
| PR | 33 (40.7) |
| CR | 14 (17.3) |
| SD | 16 (19.8) |
| DCR | 63 (77.8) |
| PD | 12 (14.8) |
| NA | 6 (7.4) |
Figure 2.
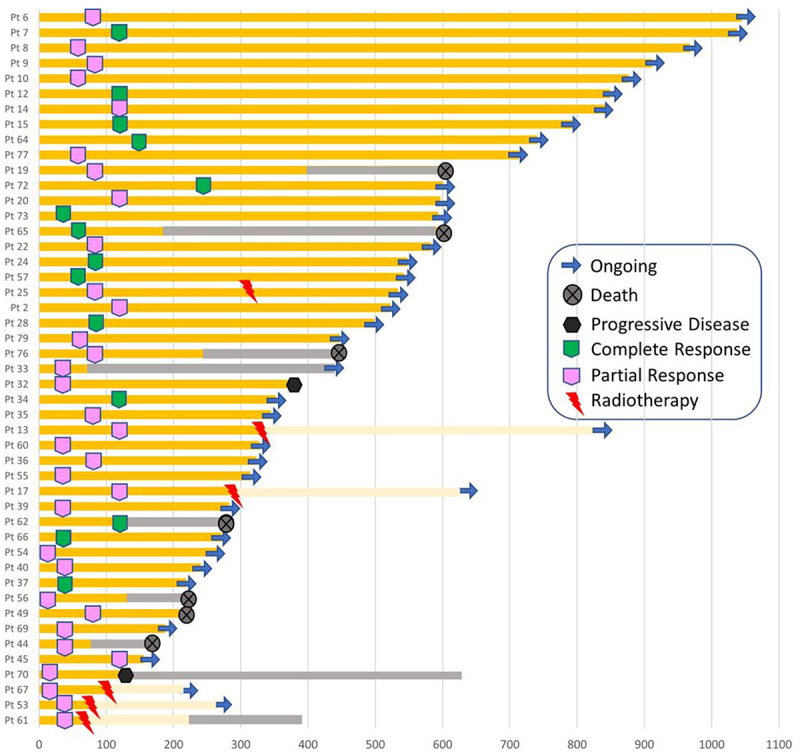
Bar graph for patients who experienced an objective response. Light yellow bars are for cemiplimab beyond-progression. Grey bars are for best supportive care (BSC).
Safety
Treatment related adverse events (TRAEs) during treatment are shown in Table 3. Percentage of TRAEs of any grade was 40.7%, while 12.3% of patients experienced a grade 3–4 TRAE. Most frequent TRAEs were skin reactions. In addition, in 15 patients (18.5%) treatment was temporarily interrupted for toxicity, of which 10 patients (12.3%) discontinued treatment definitively. No significant differences in PFS were observed between patients who did not experience toxicity compared to those who did (Supplementary Figure S3).
Table 3.
Treatment related adverse events according to CTCAE v. 5.0.
| Adverse event | Any grade (%) | Grade 3–4 (%) |
|---|---|---|
| Any | 33 (40.7) | 10 (12.3) |
| Pyrexia | 3 (3.7) | 1 (1.2) |
| Fatigue/asthenia | 2 (2.5) | 0 |
| Gastrointestinal disorders | 4 (4.9) | 0 |
| Skin reaction | 22 (27.2) | 2 (2.5) |
| Creatinine kinase elevation/myalgia | 2 (2.5) | 0 |
| Abnormal liver function | 4 (4.9) | 2 (2.5) |
| Elevated amylase and/or lipase | 1 (1.2) | 0 |
| Thyroid disfunction | 4 (4.9) | 0 |
| Pneumonitis | 1 (1.2) | 1 (1.2) |
| Acute kidney injury | 1 (1.2) | 1 (1.2) |
| Adrenal insufficiency | 3 (3.7) | 1 (1.2) |
| Adverse events leading to dose interruption | 15 (18.5) | NA |
| Adverse events leading to discontinuation of study regimen | 10 (12.3) | NA |
Gene expression profiling
In order to determine putative biomarkers of response to cemiplimab, we analyzed the activation of cancer- and immune-related genes in tumor biopsies and the surrounding microenvironment in a cohort of patients. In particular, we profiled 20 biopsies-derived RNA of pre-treated patients, of which 9 responders (R) and 11 non-responders (NR) to cemiplimab, and 3 healthy (H) biopsies-derived RNA by using Nanostring Technologies. The measurement of 770 mRNA levels describing key immuno-oncology pathways and processes provided information about tumor activity and its evasion strategies, as well as the immune cells abundance recruited in tumor sites. Interestingly, the analysis highlighted a peculiar transcriptional signature able to discriminate between R and NR patients (Figure 3a). Of note, differential expression analysis of lymphoid compartment-related genes revealed statistically significant clustering between R and NR patients, which in turn group with healthy normal tissues (χ 2 = 3.8845, p < 0.05; Figure 3b). As shown in Figure 3b, patients sensitive to cemiplimab had increased activation of the lymphoid signature before starting the treatment, while NR patients had reduced activation of immune cells and cytokines in the tumor microenvironment, similarly to what was found in the healthy donor tissues. The intersection of statistically significant de-regulated genes in R and NR patients identified 85 aberrantly expressed genes which are involved in the regulation of diverse cell processes (i.e. cell–cell adhesion, leukocyte aggregation) (Figure 4a,b). By analyzing the involved dysregulated genes, a functional network representation was created with STRING DB. Furthermore, an analysis of the involved pathways was performed with METASCAPE (Figure 4c,d). Measurement of differentially expressed gene sets between R and NR patients and healthy individual samples are summarized by a global significance score (Figure 5a). However, to better clarify the molecular mechanisms underpinning response or resistance to cemiplimab treatment, we focused on specific de-regulated genes in the two groups. In NR patient samples, the major aberrantly expressed signaling pathways were those of cell proliferation and apoptosis, as well as cytokine and chemokine signaling (Figure 5b-d). Moreover, by investigating the genes which are directly involved in the regulation of the immune system, CCL-20 and CXCL-8 were the two most frequently overexpressed chemokines in NR patients but not in R patients (Figure 5b), suggesting a potential causative role of these two genes in inducing resistance to cemiplimab.
Figure 3.
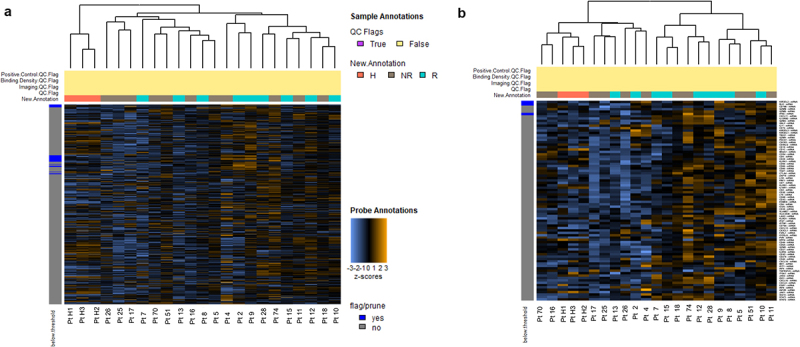
Heatmap showing unsupervised hierarchical clustering of all differentially expressed genes (a) or genes of lymphoid compartment (b) in 21 tumor samples of pre-treated patients, classified as responders (R; green line) and non-responders (NR = gray line), based on clinical outcomes measured after 3 months, and in healthy donors (H; n = 3 orange line). All the heatmaps show z-scores of differentially expressed genes. Heatmaps were generated using unsupervised clustering. Orange indicates high expression; blue indicates low expression.
Figure 4.
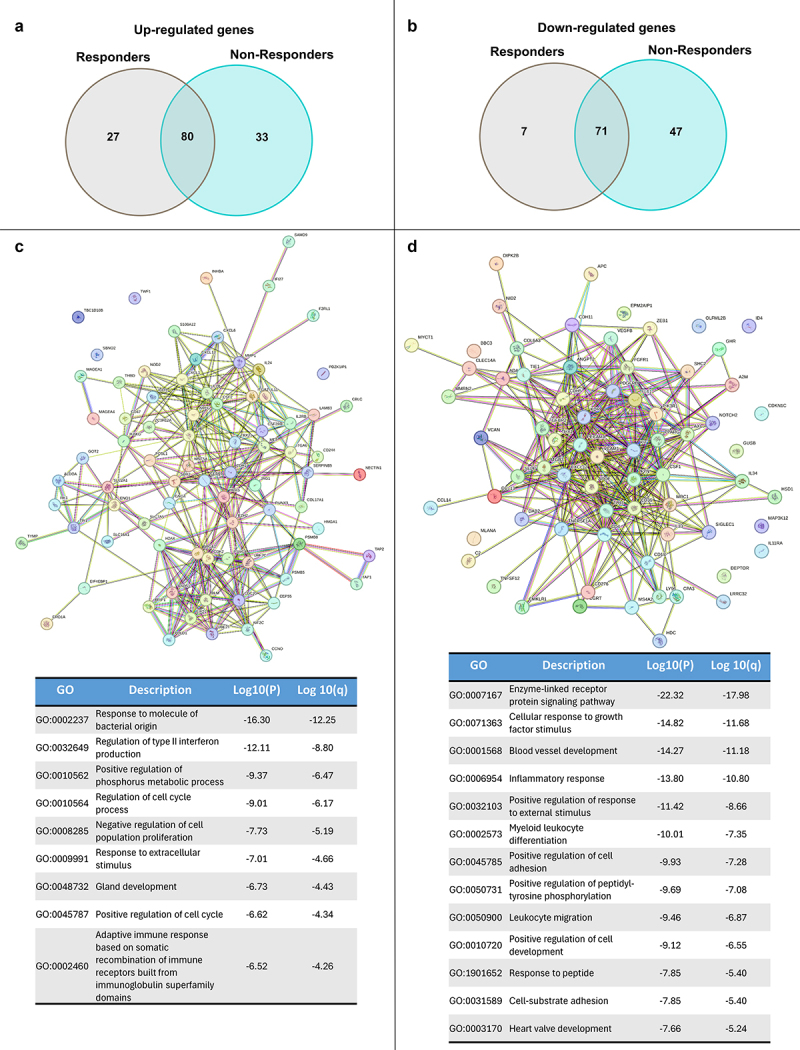
Venn-diagram of top up-regulated (a) and down-regulated (b) genes (FDR < 0.05) of responders and non-responders samples. STRING DB analysis of up-regulated (c) and down-regulated (d) genes common to responders and non-responders patients with associated (below) METASCAPE analysis and table with gene ontology (GO) annotations.
Figure 5.
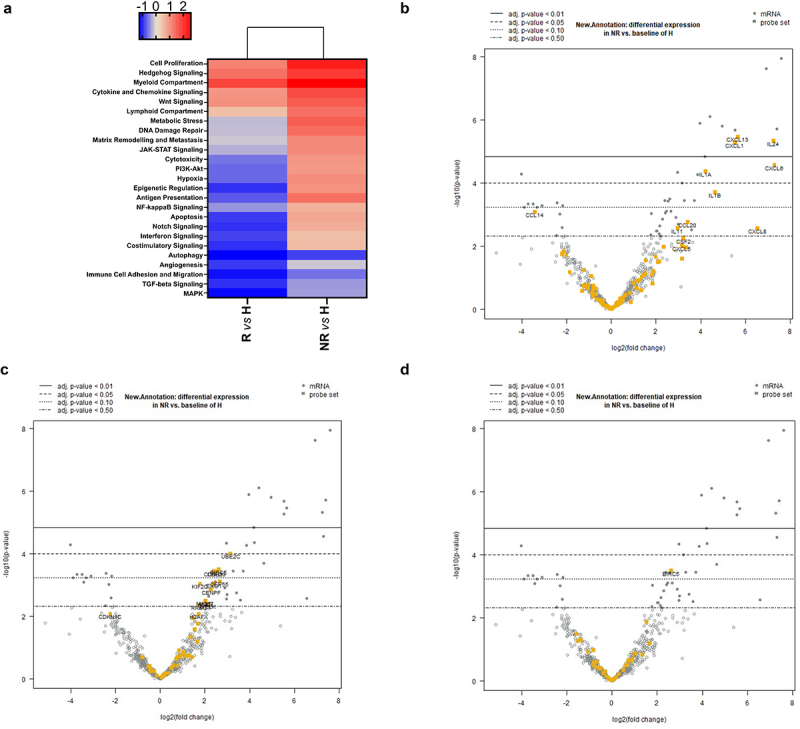
Heatmap displaying each sample’s directed global significance scores, measuring up- and down-regulated gene sets. Red denotes gene sets whose genes exhibit extensive over-expression with the covariate, blue denotes gene sets with extensive under-expression. The directed global significance score is determined as the square root of the average of the squared signed t-statistics for the genes within a gene set. These t-statistics are derived from the linear regression that forms the basis of our differential expression analysis (a). Volcano plot showing top up-regulated genes encoding for cytokines and chemokines (b), cell proliferation pathway (c), apoptosis pathway (d) in non-responders samples compared to healthy donors.
Discussion
The clinical trials leading to the approval of cemiplimab for lacSCC and mcSCC by international regulatory agencies included less than 200 patients in total.4–6 This was due both to the rarity of this pathology, which in most cases is diagnosed in the localized stage, and to the significant efficacy demonstrated by this drug. However, as it well recognized, in randomized clinical trials (RCT) some types of patients may be underrepresented, and the management of particular patients must comply with the rules of the trial.7 Our study represents one of the real-world evidence studies with the largest number of patients with lacSCC and mcSCC. Although the populations are not comparable, the results that are presented here are similar to those described in the literature with some minor differences (see Table 4). Indeed, the two prospective clinical trials involving locally advanced and metastatic patients reported response rates of 47%5 and 44%,6 respectively, as compared to 58% in the present study. The 12-months PFS was 53%5 and 58%6 in the pivotal studies and 53% in our series. If we compare our results to those of the main retrospective studies in this setting, the ORR and CR rate of our study is among the highest, although some of these patients’ cohorts are very different from each other and the treatments also include other anti-PD-1 (pembrolizumab, nivolumab).8–12
Table 4.
Data comparison between the main trials with cemiplimab in cSCC (prospective and retrospective) and our study. Cemi = Cemiplimab; Nivo = Nivolumab; Pembro = Pembrolizumab.
| Prospective Clinical Trial |
Retrospective Clinical Trial |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Migden et al., 20185 (Phase I) |
Migden et al., 20185 (Phase II) |
Migden et al., 20206 | Rischin et al., 20204 (Group 1) |
Rischin et al., 20204 (Group 3) |
Salzmann et al., 20208 | Hanna et al., 202011 | Baggi et al., 20219 | Shalhout et al., 202110 | Samaran et al., 202212 | Present study | |
| N. of patients | 26 | 59 | 78 | 59 | 56 | 46 | 61 | 131 | 76 | 63 | 81 |
| Median age (y) | 73 (55–88) | 71 (38–93) | 74 (65–81) | 71 (38–93) | 71 (38–90) | 77 (39–92) | 75 (42–95) | 79(19-95) | 74(18-98) | 83 (70–102) | 82 (48–97) |
| H&N primary(%) | 69 | 64 | 79 | 64.4 | 55.4 | 72 | 28 | 69.5 | 68 | 77.8 | 76.5 |
| mcSCC (%) | 62 | 100 | 0 | 100 | 98.2 | 87 | 77 | 30.5 | 43 | 19 | 40.7 |
| Treatment-naive (%) | 31 | 44 | 85 | 44.1 | 64.3 | 67 | 61 | 78 | 62 | NA | 100 |
| Administered treatment | Cemi | Cemi | Cemi | Cemi | Cemi | Cemi, Nivo, Pembro | Cemi, Nivo, Pembro | Cemi | Cemi, Nivo, Pembro | Cemi, Nivo, Pembro | Cemi |
| mFUP | 11.0 | 7.9 | 9.3 | 16.5 | 8.1 | NA | 8.5 | NA | NA | 8.0 | 16.4 |
| ORR (%) | 50 | 47 | 44 | 49.2 | 41.1 | 58.7 | 31.5 | 58 | 34 | 57.1 | 58 |
| CR (%) | 0 | 7 | 13 | 16.9 | 5.4 | 15.2 | 17.3 | 16 | 14 | 19 | 17.3 |
| DCR (%) | NA | NA | 79 | 71.2 | 64.3 | 80.4 | 41 | 71.7 | 70 | 68.3 | 77.8 |
| 12-months PFS rate (%) | NA | 53 | 58 | 52.9 | 47.4 | 58.8 | 55.1 for <75y 35.2 for >75y |
NA | 78 | 44.3 | 53 |
| 12-months OS rate (%) | NA | 81 | 93 | 81.3 | 76.1 | 79.3 | 46.1 | NA | 72 | 65.8 | 71 |
| TRAE rate (%) | 57.7 | 74.6 | NA | 78 | 64.3 | NA | NA | 42.7 | NA | NA | 40.7 |
| Severe TRAE rate (%) | 19.2 | 11.9 | NA | 15.3 | 12.5 | 13 | NA | 9.2 | NA | NA | 12.3 |
Another important aspect to consider in these patients is the use of surgery and/or radiotherapy. In our study, more than half of the patients had undergone surgery and about a third had received radiation therapy prior to cemiplimab treatment. Although there were no statistically significant differences between the PFS of patients receiving previous surgery and/or radiotherapy and those “naïve” to any treatment, there is a trend in favor of the former (Supplementary Figures S1a,b). This trend translates into a statistically significant difference in OS in favor of previous surgery (supplementary Figure S2a). Furthermore, contrary to some recent studies that have shown a lower probability of response in patients with previous primary surgery,9 in the series reported here the ORR of this subgroup was comparable to that of the rest of the study population (60.9% vs 58%). Furthermore, in our cohort, concomitant RT was used during treatment with cemiplimab in nine patients: in some of them, this approach has been used in tumors that are more disabling or that resulted in severe functional limitations for the patient. In fact, in four of them it was used to treat loco-regional progression and then cemiplimab was continued beyond-progression. These strategies could be clinically effective and are being evaluated in an ongoing trial.13
Overall survival data were not mature, but they are in line with the reported literature. In this population, after progression to cemiplimab, the available therapies (chemotherapy, cetuximab) are limited with scarce efficacy since rapid deterioration of patient clinical conditions generally occurs. Of note, the results of the series reported here suggest that in a subgroup of patients there is a long-term efficacy of cemiplimab treatment with survival plateau after 18–24 months.
Cemiplimab was confirmed as a safe treatment, with even a lower rate of TRAEs as compared to the pivotal trials. This may be due to the retrospective nature of our study which reduced the percentage of adverse events recorded, but also to the better management of toxicities due to the expertise we have developed over the years of using this drug. Unlike in some melanoma studies, a correlation was not observed between the experience of immune-related adverse events and survival outcome.14
In addition to clinical analyses, we also performed a transcriptional analysis on cancer tissues from 20 patients. Different landscape of immune-related genes in tumor and surrounding tissues of lacSCC and mcSCC patients may affect therapy outcomes. Indeed, gene expression data revealed that immune signatures are generally active in tumor tissues as well as in the tumor microenvironment. However, in about one-third of patients, cemiplimab treatment was not effective. Higher expression of genes involved in lymphocyte abundance, cytotoxic activity, and T-cell co-stimulatory and co-inhibitory molecules was associated to therapeutic response to cemiplimab. On the contrary, genes of the lymphoid compartment were expressed at lower levels, which were similar to those of healthy tissues in resistant patients, as compared to sensitive patients. This might negatively impact on the recruitment of immune cells with induction of inflammatory processes upon cemiplimab therapy. Moreover, non-responding tumors were characterized by high proliferative capability, as demonstrated by the over-expression of genes involved in cell cycle progression, such as UBE2C, CCNB1, CDC20, pro-proliferative genes, such as RAS (Figure 5c), and anti-apoptotic genes, such as BIRC5 (Figure 5d). Collectively, these findings suggest that differential immune-related gene activation may affect the antitumor activity of cemiplimab, thus determining therapeutic response. More specifically, we identified CCL-20 and CXCL-8, two cytokines that were up-regulated in patients which were resistant to cemiplimab treatment, as potential biomarkers of lack of therapeutic efficacy. It has been shown that these cytokines may promote cancer invasion and migration, stemness, and epithelial – mesenchymal transition (EMT).15,16 Moreover, CCL20 allows recruitment of regulatory T cells (Tregs) into the tumor tissue in colorectal cancer.17 Rutihinda et al. have demonstrated that radiotherapy promotes the infiltration of T-regulatory cells (Treg) via CCL-20 and that the inhibition of CCL-20 can enhance the response to RT in head and neck squamous cancers (HNC).18 Furthermore, several studies have shown increased expression of this chemokine in pre-cancerous skin lesions and in cSCC samples.19,20 CXCL-8, also known as IL-8, induces Tregs migration prompting tumor escape21 and has a key role in tolerogenic myeloid-cell infiltration in the tumor microenvironment.22 Moreover, it has been shown that an increase in IL-8 levels, in the serum of lung cancer and melanoma patients treated with immune-checkpoint inhibitors, is a predictor of poor outcome, suggesting a role for this chemokine in immune resistance.23 A detrimental survival effect of basal IL-8 circulating levels was confirmed in a cohort of 1344 patients from four phase III clinical trials, including patients with lung cancer, kidney cancer and melanoma, that were treated with nivolumab and/or ipilimumab.22 However, little is known on the prognostic and predictive roles of IL-8 and CCL-20 in cSCC. Instead, a recent work by Mallardo et al., has shown that serum levels of IL-6 in cSCC patients after treatment with cemiplimab are associated with a worse response to therapy.24 Thus, the negative immunomodulatory effect of these cytokines could, at least in part, explain resistance to immune checkpoint inhibition.
The present study has some limitations, which mainly concern with the retrospective nature and with the relative heterogeneity of the patient population. Furthermore, the gene expression profiling has been done on a limited number of cases and, therefore, allows only for generating hypothesis. We do not have data on the actual protein expression of these dysregulated genes: a prospective study on sera from patients undergoing cemiplimab therapy is ongoing. Further prospective studies with an adequate number of patients and of tumor tissues are needed to confirm these initial findings.
Supplementary Material
Acknowledgments
We thank the Southern Italy Oncology Group (GOIM) for the scientific support.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
S.N. had travel grants from Amgen, Merck outside of the submitted works. D.C. had travel support from Sanofi, BMS, Merck serono outside of the submitted works. F.C. was advisory board for Amgen, Servier, MSD, Merck, Roche, Pfizer, Bayer, Pierre Fabre, Eisai outside of the submitted work. T.T. was advisory board for Amgen, MSD, Pierre Fabre, Roche, Merck outside of the submitted work. All remaining authors have no competing interests.
Authors’ contributions
Conceptualization: VDF, SN, LF, TT; data curation: VDF, GS, RN, AE, FC, MCG, EC, VF; formal analysis: VDF, FMZ, DE, VF; investigation; VDF, GS, RN, AE, FC, MCG, EC; project administration: FC, RF, RB, GA; supervision: FC, RF, RB, GA; visualization: all authors; writing-original draft: VDF, SN, TT, LF, DE; writing-review and editing: SN,TT, LF, FC.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The retrospective study protocol was approved by the institutional review board at the main study site (Università della Campania “Luigi Vanvitelli”, Protocol n°59). The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients signed a written informed consent and agreed with the research use of their anonymized data.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/2162402X.2024.2388315
References
- 1.Stratigos AJ, Garbe C, Dessinioti C, Lebbe C, Bataille V, Bastholt L, Dreno B, Fargnoli MC, Forsea AM, Frenard C, et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: part 1. epidemiology, diagnostics and prevention. Eur J Cancer. 2020;128:60–11. doi: 10.1016/j.ejca.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Jarkowski A, Hare R, Loud P, Skitzki JJ, Kane JM, May KS, Zeitouni NC, Nestico J, Vona KL, Groman A, et al. Systemic therapy in advanced cutaneous squamous cell carcinoma (CSCC). Am J Clin Oncol. 2016;39(6):545–548. doi: 10.1097/COC.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 3.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale A-L, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rischin D, Migden MR, Lim AM, Schmults CD, Khushalani NI, Hughes BGM, Schadendorf D, Dunn LA, Hernandez-Aya L, Chang ALS, et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J Immunother Cancer. 2020;8(1):e000775. doi: 10.1136/jitc-2020-000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, Chung CH, Hernandez-Aya L, Lim AM, Chang ALS, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 6.Migden MR, Khushalani NI, Chang ALS, Lewis KD, Schmults CD, Hernandez-Aya L, Meier F, Schadendorf D, Guminski A, Hauschild A, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020;21(2):294–305. doi: 10.1016/S1470-2045(19)30728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Maio M, Perrone F, Conte P.. Real-world evidence in oncology: opportunities and limitations. Oncologist. 2020;25(5):e746–e752. doi: 10.1634/theoncologist.2019-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salzmann M, Leiter U, Loquai C, Zimmer L, Ugurel S, Gutzmer R, Thoms K-M, Enk AH, Hassel JC. Programmed cell death protein 1 inhibitors in advanced cutaneous squamous cell carcinoma: real-world data of a retrospective, multicenter study. Eur J Cancer. 2020;138:125–132. doi: 10.1016/j.ejca.2020.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Baggi A, Quaglino P, Rubatto M, Depenni R, Guida M, Ascierto PA, Trojaniello C, Queirolo P, Saponara M, Peris K, et al. Real world data of cemiplimab in locally advanced and metastatic cutaneous squamous cell carcinoma. Eur J Cancer. 2021;157:250–258. doi: 10.1016/j.ejca.2021.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Shalhout SZ, Park JC, Emerick KS, Sullivan RJ, Kaufman HL, Miller DM. Real-world assessment of response to anti-programmed cell death 1 therapy in advanced cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2021;85(4):1038–1040. doi: 10.1016/j.jaad.2021.01.048. [DOI] [PubMed] [Google Scholar]
- 11.Hanna GJ, Ruiz ES, LeBoeuf NR, Thakuria M, Schmults CD, Decaprio JA, Silk AW. Real-world outcomes treating patients with advanced cutaneous squamous cell carcinoma with immune checkpoint inhibitors (CPI). Br J Cancer. 2020;123(10):1535–1542. doi: 10.1038/s41416-020-01044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samaran Q, Samaran R, Ferreira E, Haddad N, Fottorino A, Maillard H, Dreno B, Meyer N, Azria D, Maubec E, et al. Anti-PD-1 for the treatment of advanced cutaneous squamous cell carcinoma in elderly patients: a French multicenter retrospective survey. J Cancer Res Clin Oncol. 2023;149(7):3549–3562. doi: 10.1007/s00432-022-04246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin C, Ballah T, Nottage M, Hay K, Chua B, Kenny L, Thomas P, Teng M, Keller J, Le T, et al. A prospective study investigating the efficacy and toxicity of definitive ChemoRadiation and ImmunOtherapy (CRIO) in locally and/or regionally advanced unresectable cutaneous squamous cell carcinoma. Radiat Oncol. 2021;16(1):69. doi: 10.1186/s13014-021-01795-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serna-Higuita LM, Amaral T, Forschner A, Leiter U, Flatz L, Seeber O, Thomas I, Garbe C, Eigentler TK, Martus P. Association between immune-related adverse events and survival in 319 stage IV melanoma patients treated with PD-1-Based immunotherapy: an approach based on clinical chemistry. Cancers (Basel). 2021;13(23):6141. doi: 10.3390/cancers13236141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559–572. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hippe A, Braun SA, Oláh P, Gerber PA, Schorr A, Seeliger S, Holtz S, Jannasch K, Pivarcsi A, Buhren B, et al. Egfr/ras-induced CCL20 production modulates the tumour microenvironment. Br J Cancer. 2020;123(6):942–954. doi: 10.1038/s41416-020-0943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Yang L, Yu W, Wu Q, Lian J, Li F, Liu S, Li A, He Z, Liu J, et al. Colorectal cancer cell-derived CCL20 recruits regulatory T cells to promote chemoresistance via FOXO1/CEBPB/NF-κB signaling. J Immunother Cancer. 2019;7(1):215. doi: 10.1186/s40425-019-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rutihinda C, Haroun R, Saidi NE, Ordoñez JP, Naasri S, Lévesque D, Boisvert F-M, Fortier P-H, Belzile M, Fradet L, et al. Inhibition of the CCR6-CCL20 axis prevents regulatory T cell recruitment and sensitizes head and neck squamous cell carcinoma to radiation therapy. Cancer Immunol, Immunother. 2023;72(5):1089–1102. doi: 10.1007/s00262-022-03313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuong ZK, Lewandowski A, Bridge JA, Cruz JLG, Yamada M, Lambie D, Lewandowski R, Steptoe RJ, Leggatt GR, Simpson F, et al. Cytokine/Chemokine profiles in squamous cell carcinoma correlate with precancerous and cancerous disease stage. Sci Rep. 2019;9(1):17754. doi: 10.1038/s41598-019-54435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato Y, Fujimura T, Hidaka T, Lyu C, Tanita K, Matsushita S, Yamamoto M, Aiba S. Possible roles of proinflammatory signaling in keratinocytes through aryl hydrocarbon receptor ligands for the development of squamous cell carcinoma. Front Immunol. 2020;11:11. doi: 10.3389/fimmu.2020.534323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang S-P, Walsh AM, Baxi V, Pandya D, Baradet T, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. 2020;26(5):688–692. doi: 10.1038/s41591-020-0856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eikawa S, Ohue Y, Kitaoka K, Aji T, Uenaka A, Oka M, Nakayama E. Enrichment of Foxp3+ CD4 regulatory T cells in migrated T cells to IL-6– and IL-8–expressing tumors through predominant induction of CXCR1 by IL-6. J Immunol. 2010;185(11):6734–6740. doi: 10.4049/jimmunol.1000225. [DOI] [PubMed] [Google Scholar]
- 23.Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, Oñate C, Perez G, Alfaro C, Martín-Algarra S. et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol. 2017;28(8):1988–1995. doi: 10.1093/annonc/mdx190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mallardo D, Simeone E, Festino L, Tuffanelli M, Vanella V, Trojaniello C, Vitale MG, Ottaviano M, Capone M, Madonna G, et al. IL-6 as new prognostic factor in patients with advanced cutaneous squamous cell carcinoma treated with cemiplimab. J Transl Med. 2023;21(1):140. doi: 10.1186/s12967-023-03971-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


