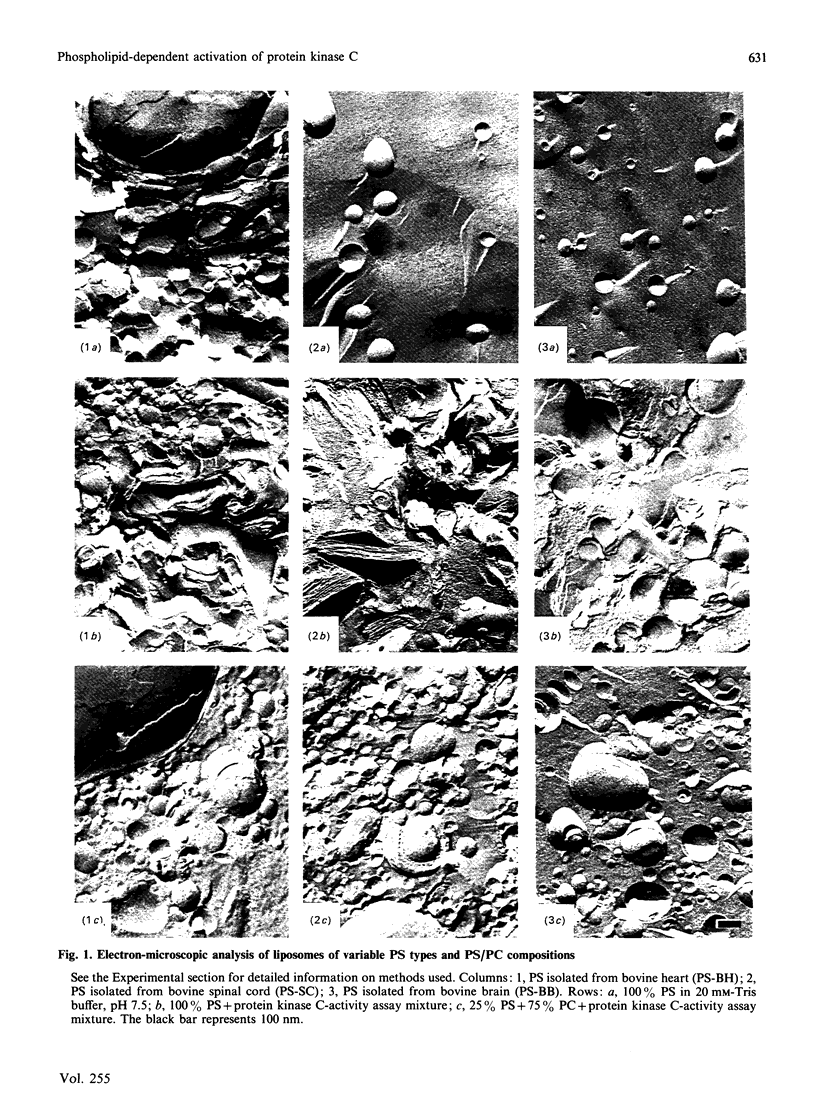
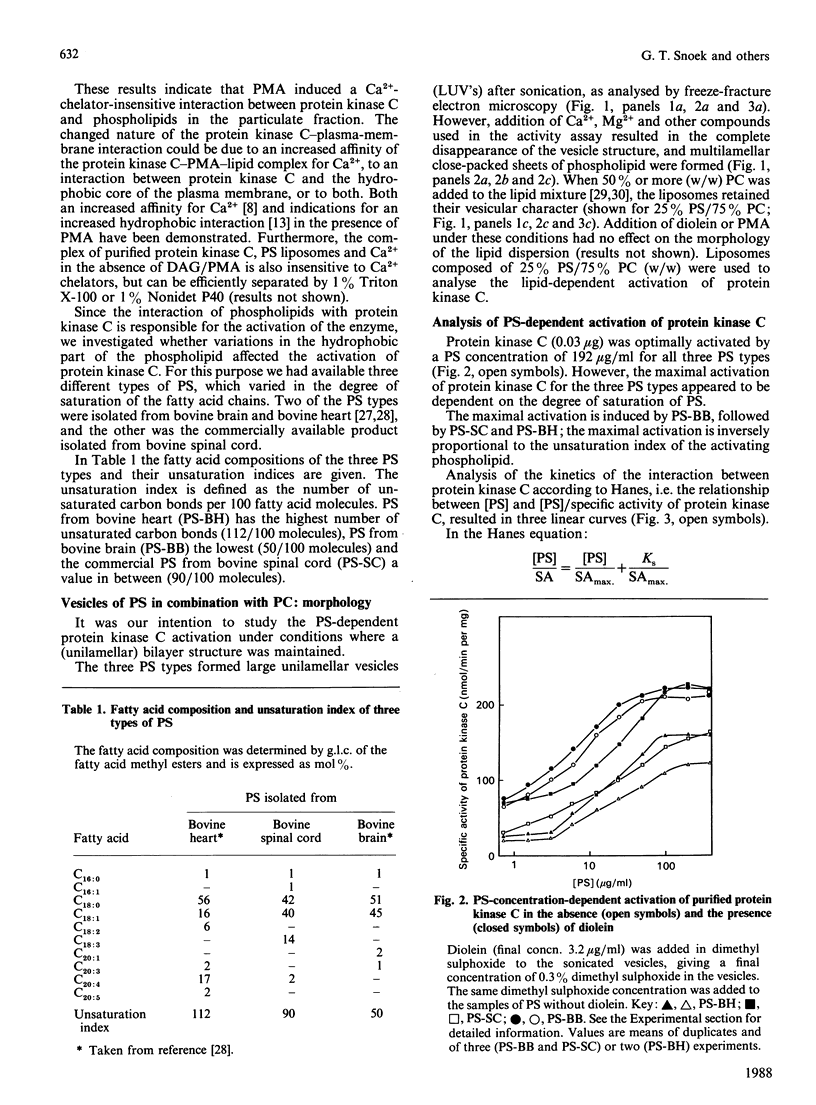
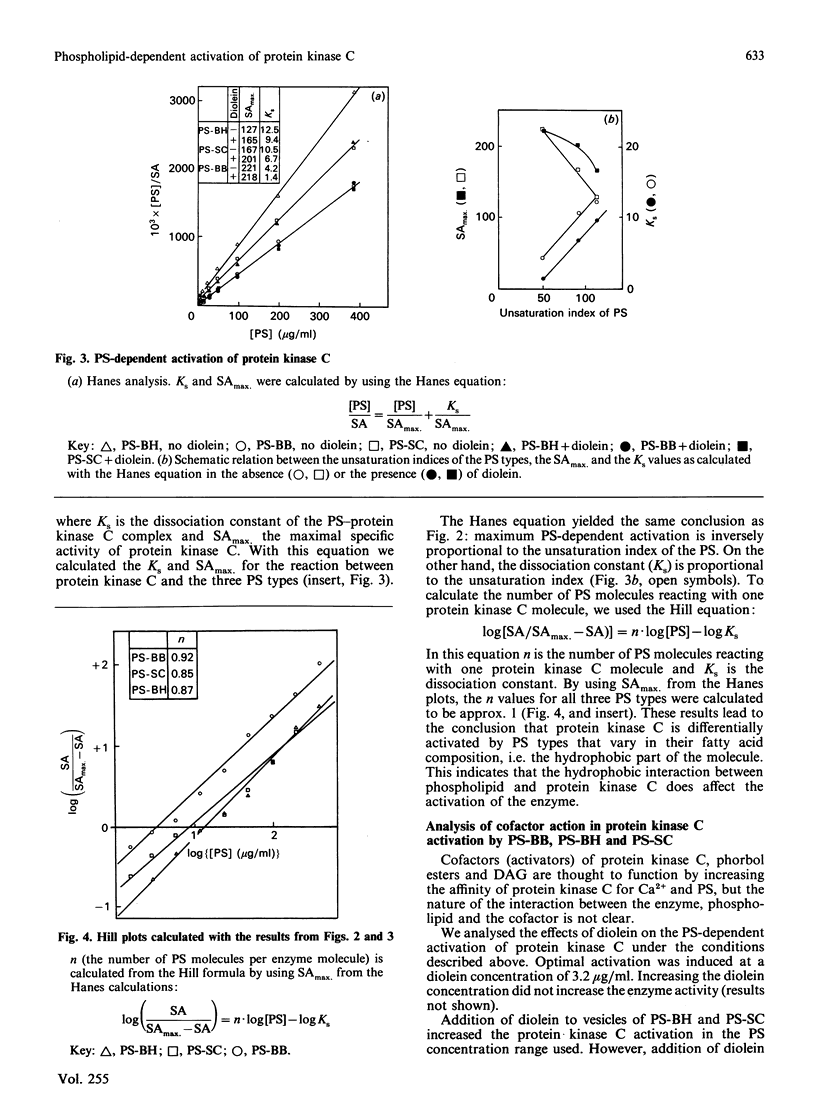
Abstract
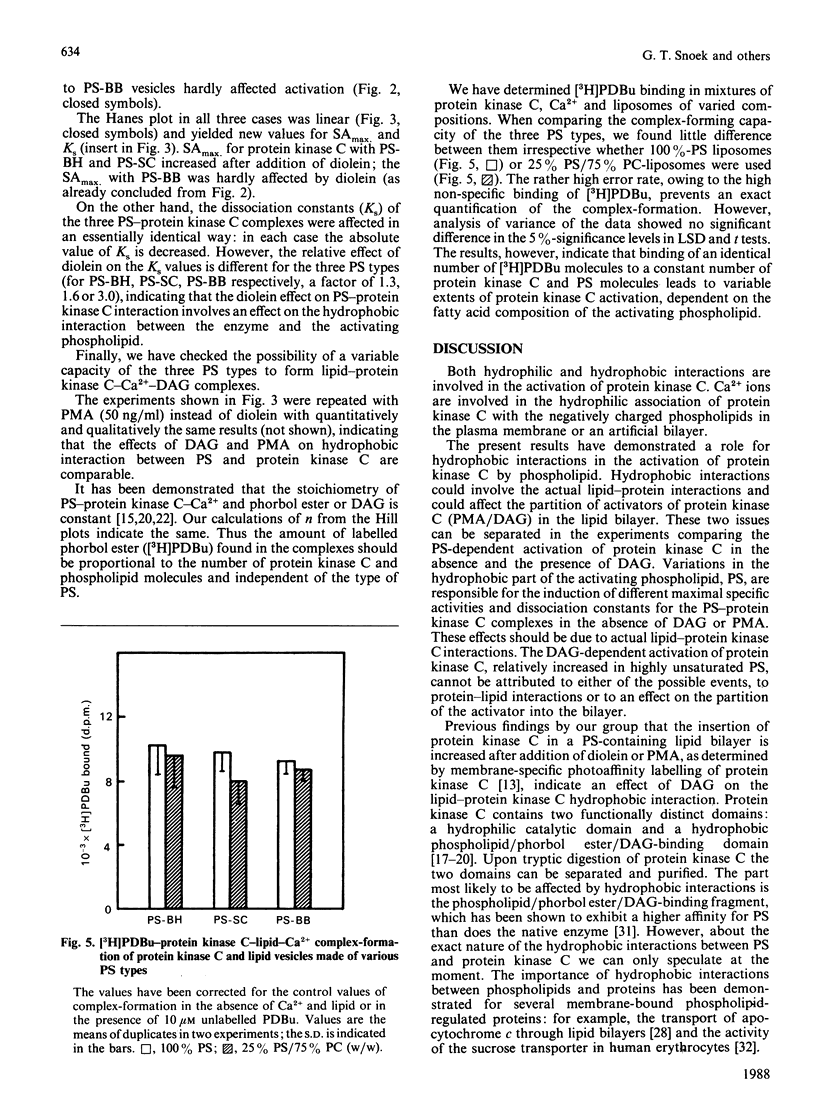
The effects of hydrophobic interaction on the activation of Ca2+-stimulated phospholipid-dependent protein kinase (protein kinase C), isolated from mouse brain, by phosphatidylserine (PS) and diacylglycerol (DAG) or phorbol 12-myristate 13-acetate were studied. To maintain bilayer structure during assay conditions, phosphatidylcholine was added to the PS vesicles. The vesicular structure of all types of PS was confirmed by freeze-fracture electron microscopy. The PS-dependent activation of purified protein kinase C from mouse brain is affected by the fatty acid composition of PS: an inverse relationship between the unsaturation index of PS (isolated from bovine heart, bovine spinal cord or bovine brain) and the ability to activate protein kinase C was demonstrated. In highly saturated PS lipid dispersions, only slight additional activation of protein kinase C by DAG was found, in contrast with highly unsaturated PS lipid dispersion, where DAG increased protein kinase C activity by 2-3-fold at optimal PS concentrations. We quantified the formation of the protein kinase C-Ca2+-PS-phorbol ester complex by using [3H]phorbol 12,13-dibutyrate [( 3H]PDBu). The efficiency of complex-formation, determined as the amount of [3H]PDBu bound, is not affected by variations in the hydrophobic part of PS. These results indicate a role of the hydrophobic part of the activating phospholipid in the activation mechanism of protein kinase C and in the action of cofactors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. M. Protein kinase C activation by diacylglycerol second messengers. Cell. 1986 Jun 6;45(5):631–632. doi: 10.1016/0092-8674(86)90774-9. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Jaken S., König B., Sharkey N. A., Leach K. L., Jeng A. Y., Yeh E. Mechanism of action of the phorbol ester tumor promoters: specific receptors for lipophilic ligands. Biochem Pharmacol. 1984 Mar 15;33(6):933–940. doi: 10.1016/0006-2952(84)90448-9. [DOI] [PubMed] [Google Scholar]
- Boni L. T., Rando R. R. The nature of protein kinase C activation by physically defined phospholipid vesicles and diacylglycerols. J Biol Chem. 1985 Sep 5;260(19):10819–10825. [PubMed] [Google Scholar]
- Cabot M. C. Effect of cellular phospholipid modification on phorbol diester binding. Cancer Res. 1983 Sep;43(9):4233–4238. [PubMed] [Google Scholar]
- Cabot M. C., Jaken S. Structural and chemical specificity of diacylglycerols for protein kinase C activation. Biochem Biophys Res Commun. 1984 Nov 30;125(1):163–169. doi: 10.1016/s0006-291x(84)80349-6. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Coussens L., Parker P. J., Rhee L., Yang-Feng T. L., Chen E., Waterfield M. D., Francke U., Ullrich A. Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science. 1986 Aug 22;233(4766):859–866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- Davis R. J., Ganong B. R., Bell R. M., Czech M. P. Structural requirements for diacylglycerols to mimic tumor-promoting phobol diester action on the epidermal growth factor receptor. J Biol Chem. 1985 May 10;260(9):5315–5322. [PubMed] [Google Scholar]
- Düzgüneş N., Wilschut J., Fraley R., Papahadjopoulos D. Studies on the mechanism of membrane fusion. Role of head-group composition in calcium- and magnesium-induced fusion of mixed phospholipid vesicles. Biochim Biophys Acta. 1981 Mar 20;642(1):182–195. doi: 10.1016/0005-2736(81)90148-6. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Anderson W. B. Interleukin-2 stimulates association of protein kinase C with plasma membrane. Nature. 1985 May 16;315(6016):233–235. doi: 10.1038/315233a0. [DOI] [PubMed] [Google Scholar]
- Ganong B. R., Loomis C. R., Hannun Y. A., Bell R. M. Specificity and mechanism of protein kinase C activation by sn-1,2-diacylglycerols. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1184–1188. doi: 10.1073/pnas.83.5.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard P. R., Mazzei G. J., Kuo J. F. Immunological quantitation of phospholipid/Ca2+-dependent protein kinase and its fragments. Tissue levels, subcellular distribution, and ontogenetic changes in brain and heart. J Biol Chem. 1986 Jan 5;261(1):370–375. [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Bell R. M. Activation of protein kinase C by Triton X-100 mixed micelles containing diacylglycerol and phosphatidylserine. J Biol Chem. 1985 Aug 25;260(18):10039–10043. [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Bell R. M. Protein kinase C activation in mixed micelles. Mechanistic implications of phospholipid, diacylglycerol, and calcium interdependencies. J Biol Chem. 1986 Jun 5;261(16):7184–7190. [PubMed] [Google Scholar]
- Hokin L. E. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Hoshijima M., Kikuchi A., Tanimoto T., Kaibuchi K., Takai Y. Formation of a phorbol ester-binding fragment from protein kinase C by proteolytic digestion. Cancer Res. 1986 Jun;46(6):3000–3004. [PubMed] [Google Scholar]
- Huang K. P., Huang F. L. Conversion of protein kinase C from a Ca2+-dependent to an independent form of phorbol ester-binding protein by digestion with trypsin. Biochem Biophys Res Commun. 1986 Aug 29;139(1):320–326. doi: 10.1016/s0006-291x(86)80116-4. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Nishizuka Y. Cooperative roles of various membrane phospholipids in the activation of calcium-activated, phospholipid-dependent protein kinase. J Biol Chem. 1981 Jul 25;256(14):7146–7149. [PubMed] [Google Scholar]
- Kishimoto A., Kajikawa N., Shiota M., Nishizuka Y. Proteolytic activation of calcium-activated, phospholipid-dependent protein kinase by calcium-dependent neutral protease. J Biol Chem. 1983 Jan 25;258(2):1156–1164. [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Reep B., Ganong B. R., Bell R. M. Exogenous sn-1,2-diacylglycerols containing saturated fatty acids function as bioregulators of protein kinase C in human platelets. J Biol Chem. 1985 Feb 10;260(3):1358–1361. [PubMed] [Google Scholar]
- Leach K. L., James M. L., Blumberg P. M. Characterization of a specific phorbol ester aporeceptor in mouse brain cytosol. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4208–4212. doi: 10.1073/pnas.80.14.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Portis A., Pangborn W. Calcium-induced lipid phase transitions and membrane fusion. Ann N Y Acad Sci. 1978;308:50–66. doi: 10.1111/j.1749-6632.1978.tb22013.x. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Stabel S., Waterfield M. D. Purification to homogeneity of protein kinase C from bovine brain--identity with the phorbol ester receptor. EMBO J. 1984 May;3(5):953–959. doi: 10.1002/j.1460-2075.1984.tb01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld A., Jordi W., de Kruijff B. Studies on the lipid dependency and mechanism of the translocation of the mitochondrial precursor protein apocytochrome c across model membranes. J Biol Chem. 1986 Mar 15;261(8):3846–3856. [PubMed] [Google Scholar]
- Shoyab M. Long-chain fatty acyl-coenzyme A's activate both the ligand-binding and protein kinase activities of phorboid and ingenoid receptor. Arch Biochem Biophys. 1985 Jan;236(1):435–440. doi: 10.1016/0003-9861(85)90644-7. [DOI] [PubMed] [Google Scholar]
- Snoek G. T., Mummery C. L., van den Brink C. E., van der Saag P. T., de Laat S. W. Protein kinase C and phorbol ester receptor expression related to growth and differentiation of nullipotent and pluripotent embryonal carcinoma cells. Dev Biol. 1986 Jun;115(2):282–292. doi: 10.1016/0012-1606(86)90249-6. [DOI] [PubMed] [Google Scholar]
- Snoek G. T., Rosenberg I., de Laat S. W., Gitler C. The interaction of protein kinase C and other specific cytoplasmic proteins with phospholipid bilayers. Biochim Biophys Acta. 1986 Aug 21;860(2):336–344. doi: 10.1016/0005-2736(86)90530-4. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Miyake R., Kikkawa U., Nishizuka Y. Rapid assay of binding of tumor-promoting phorbol esters to protein kinase C1. J Biochem. 1986 Jan;99(1):257–261. doi: 10.1093/oxfordjournals.jbchem.a135467. [DOI] [PubMed] [Google Scholar]
- Tapley P. M., Murray A. W. Platelet Ca2+-activated, phospholipid-dependent protein kinase: evidence for proteolytic activation of the enzyme in cells treated with phospholipase C1. Biochem Biophys Res Commun. 1984 Feb 14;118(3):835–841. doi: 10.1016/0006-291x(84)91470-0. [DOI] [PubMed] [Google Scholar]
- van Dijck P. W., de Kruijff B., Verkleij A. J., van Deenen L. L., de Gier J. Comparative studies on the effects of pH and Ca2+ on bilayers of various negatively charged phospholipids and their mixtures with phosphatidylcholine. Biochim Biophys Acta. 1978 Sep 11;512(1):84–96. doi: 10.1016/0005-2736(78)90219-5. [DOI] [PubMed] [Google Scholar]