Abstract
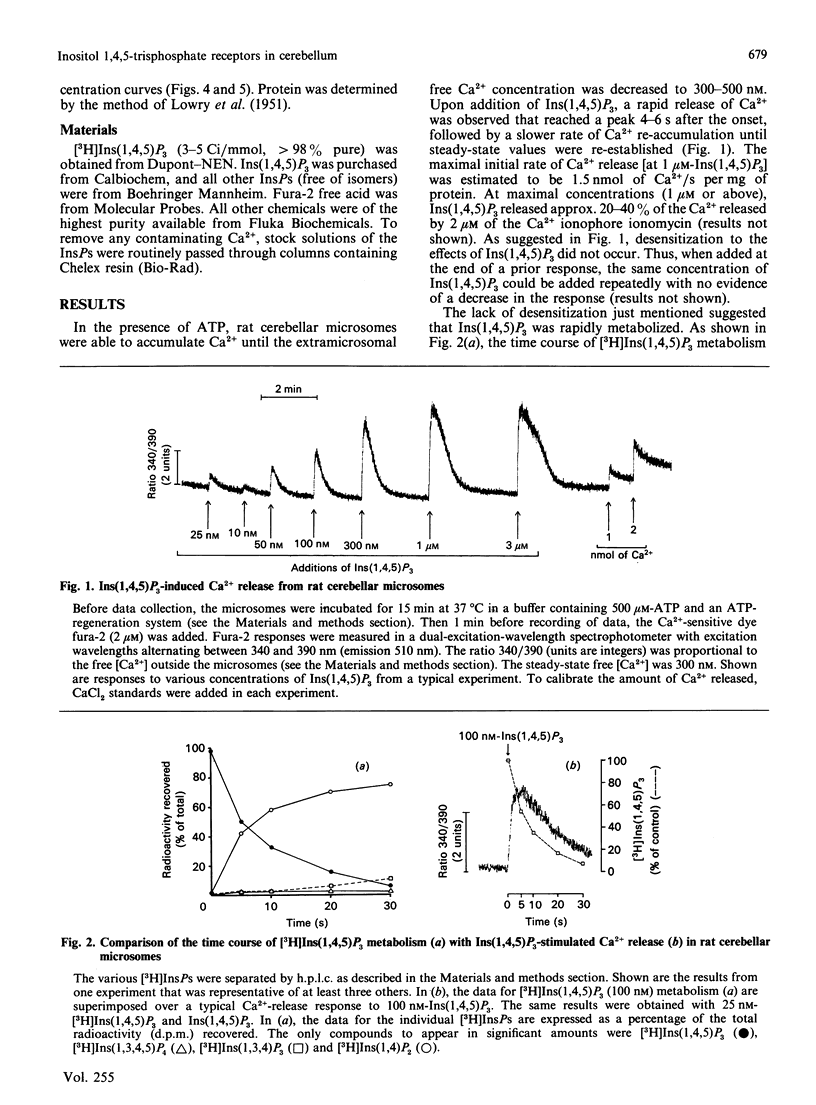
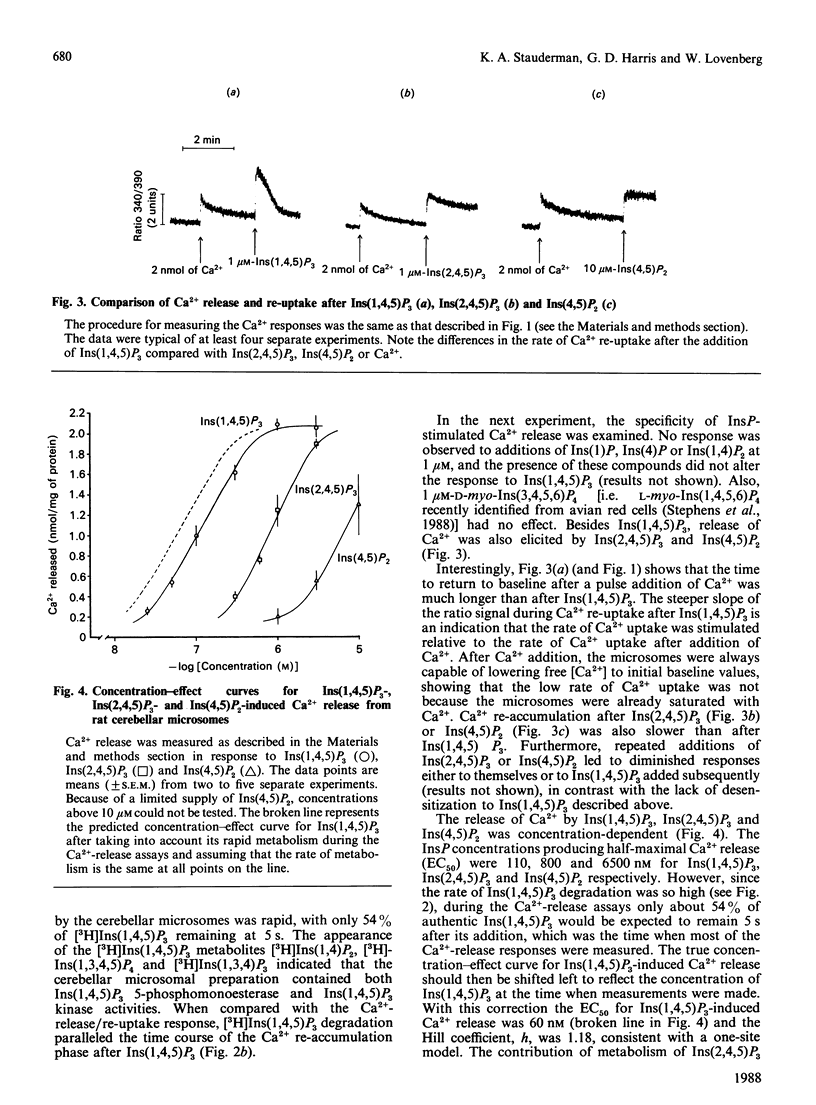
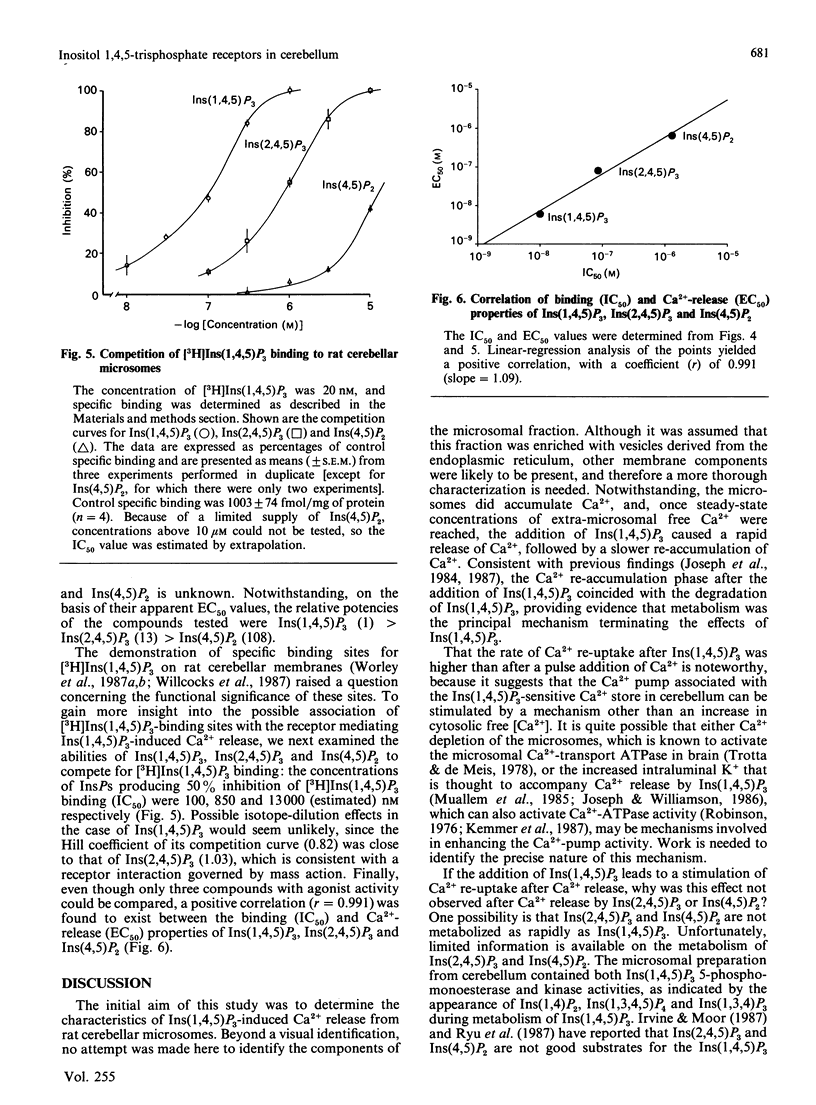
The abilities of D-myo-inositol phosphates (InsPs) to promote Ca2+ release and to compete for D-myo-[3H]-inositol 1,4,5-trisphosphate [( 3H]Ins(1,4,5)P3) binding were examined with microsomal preparations from rat cerebellum. Of the seven InsPs examined, only Ins(1,4,5)P3, Ins(2,4,5)P3 and Ins(4,5)P2 stimulated the release of Ca2+. Ca2+ release was maximal in 4-6 s and was followed by a rapid re-accumulation of Ca2+ into the Ins(1,4,5)P3-sensitive compartment after Ins(1,4,5)P3, but not after Ins(2,4,5)P3 or Ins(4,5)P2. Ca2+ re-accumulation after Ins(1,4,5)P3 was also faster than after pulse additions of Ca2+, and coincided with the metabolism of [3H]Ins(1,4,5)P3. These data suggest that Ins(1,4,5)P3-induced Ca2+ release and the accompanying decrease in intraluminal Ca2+ stimulate the Ca2+ pump associated with the Ins(1,4,5)P3-sensitive compartment. That this effect was observed only after Ins(1,4,5)P3 may reflect differences in either the metabolic rates of the various InsPs or an effect of the Ins(1,4,5)P3 metabolite Ins(1,3,4,5)P4 to stimulate refilling of the Ins(1,4,5)P3-sensitive store. InsP-induced Ca2+ release was concentration-dependent, with EC50 values (concn. giving half-maximal release) of 60, 800 and 6500 nM for Ins(1,4,5)P3, Ins(2,4,5)P3 and Ins(4,5)P2 respectively. Ins(1,4,5)P3, Ins(2,4,5)P3 and Ins(4,5)P2 also competed for [3H]Ins(1,4,5)P3 binding, with respective IC50 values (concn. giving 50% inhibition) of 100, 850 and 13,000 nM. Comparison of the EC50 and IC50 values yielded a significant correlation (r = 0.991). These data provide evidence of an association between the [3H]Ins(1,4,5)P3-binding site and the receptor mediating Ins(1,4,5)P3-induced Ca2+ release.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baukal A. J., Guillemette G., Rubin R., Spät A., Catt K. J. Binding sites for inositol trisphosphate in the bovine adrenal cortex. Biochem Biophys Res Commun. 1985 Dec 17;133(2):532–538. doi: 10.1016/0006-291x(85)90939-8. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Irvine R. F., Berridge M. J., McKinney J. S., Putney J. W., Jr Actions of inositol phosphates on Ca2+ pools in guinea-pig hepatocytes. Biochem J. 1984 Dec 15;224(3):741–746. doi: 10.1042/bj2240741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Irvine R. F. Inositol (1,4,5)trisphosphate-promoted Ca2+ release from microsomal fractions of rat liver. Biochem Biophys Res Commun. 1984 May 16;120(3):858–864. doi: 10.1016/s0006-291x(84)80186-2. [DOI] [PubMed] [Google Scholar]
- Fisher S. K., Agranoff B. W. Receptor activation and inositol lipid hydrolysis in neural tissues. J Neurochem. 1987 Apr;48(4):999–1017. doi: 10.1111/j.1471-4159.1987.tb05618.x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Guillemette G., Balla T., Baukal A. J., Spät A., Catt K. J. Intracellular receptors for inositol 1,4,5-trisphosphate in angiotensin II target tissues. J Biol Chem. 1987 Jan 25;262(3):1010–1015. [PubMed] [Google Scholar]
- Hansen C. A., Mah S., Williamson J. R. Formation and metabolism of inositol 1,3,4,5-tetrakisphosphate in liver. J Biol Chem. 1986 Jun 25;261(18):8100–8103. [PubMed] [Google Scholar]
- Irvine R. F., Brown K. D., Berridge M. J. Specificity of inositol trisphosphate-induced calcium release from permeabilized Swiss-mouse 3T3 cells. Biochem J. 1984 Aug 15;222(1):269–272. doi: 10.1042/bj2220269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Inositol(1,3,4,5)tetrakisphosphate-induced activation of sea urchin eggs requires the presence of inositol trisphosphate. Biochem Biophys Res Commun. 1987 Jul 15;146(1):284–290. doi: 10.1016/0006-291x(87)90723-6. [DOI] [PubMed] [Google Scholar]
- Jean T., Klee C. B. Calcium modulation of inositol 1,4,5-trisphosphate-induced calcium release from neuroblastoma x glioma hybrid (NG108-15) microsomes. J Biol Chem. 1986 Dec 15;261(35):16414–16420. [PubMed] [Google Scholar]
- Joseph S. K., Hansen C. A., Williamson J. R. Inositol 1,3,4,5-tetrakisphosphate increases the duration of the inositol 1,4,5-trisphosphate-mediated Ca2+ transient. FEBS Lett. 1987 Jul 13;219(1):125–129. doi: 10.1016/0014-5793(87)81203-6. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Joseph S. K., Williamson J. R. Characteristics of inositol trisphosphate-mediated Ca2+ release from permeabilized hepatocytes. J Biol Chem. 1986 Nov 5;261(31):14658–14664. [PubMed] [Google Scholar]
- Kemmer T. P., Bayerdörffer E., Will H., Schulz I. Anion dependence of Ca2+ transport and (Ca2+ + K+)-stimulated Mg2+-dependent transport ATPase in rat pancreatic endoplasmic reticulum. J Biol Chem. 1987 Oct 5;262(28):13758–13764. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meyer T., Holowka D., Stryer L. Highly cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988 Apr 29;240(4852):653–656. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Muallem S., Schoeffield M., Pandol S., Sachs G. Inositol trisphosphate modification of ion transport in rough endoplasmic reticulum. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4433–4437. doi: 10.1073/pnas.82.13.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Prentki M., Biden T. J., Janjic D., Irvine R. F., Berridge M. J., Wollheim C. B. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature. 1984 Jun 7;309(5968):562–564. doi: 10.1038/309562a0. [DOI] [PubMed] [Google Scholar]
- Robinson J. D. (Ca + Mg)-stimulated ATPase activity of a rat brain microsomal preparation. Arch Biochem Biophys. 1976 Sep;176(1):366–374. doi: 10.1016/0003-9861(76)90176-4. [DOI] [PubMed] [Google Scholar]
- Ryu S. H., Lee S. Y., Lee K. Y., Rhee S. G. Catalytic properties of inositol trisphosphate kinase: activation by Ca2+ and calmodulin. FASEB J. 1987 Nov;1(5):388–393. doi: 10.1096/fasebj.1.5.2824270. [DOI] [PubMed] [Google Scholar]
- Spät A., Bradford P. G., McKinney J. S., Rubin R. P., Putney J. W., Jr A saturable receptor for 32P-inositol-1,4,5-triphosphate in hepatocytes and neutrophils. Nature. 1986 Feb 6;319(6053):514–516. doi: 10.1038/319514a0. [DOI] [PubMed] [Google Scholar]
- Spät A., Fabiato A., Rubin R. P. Binding of inositol trisphosphate by a liver microsomal fraction. Biochem J. 1986 Feb 1;233(3):929–932. doi: 10.1042/bj2330929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spät A., Lukács G. L., Eberhardt I., Kiesel L., Runnebaum B. Binding of inositol phosphates and induction of Ca2+ release from pituitary microsomal fractions. Biochem J. 1987 Jun 1;244(2):493–496. doi: 10.1042/bj2440493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L., Hawkins P. T., Carter N., Chahwala S. B., Morris A. J., Whetton A. D., Downes P. C. L-myo-inositol 1,4,5,6-tetrakisphosphate is present in both mammalian and avian cells. Biochem J. 1988 Jan 1;249(1):271–282. doi: 10.1042/bj2490271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H., Bayerdörffer E., Haase W., Irvine R. F., Schulz I. Effect of inositol-1,4,5-trisphosphate on isolated subcellular fractions of rat pancreas. J Membr Biol. 1984;81(3):241–253. doi: 10.1007/BF01868717. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Tennes K. A., McKinney J. S., Putney J. W., Jr Metabolism of inositol 1,4,5-trisphosphate in guinea-pig hepatocytes. Biochem J. 1987 Mar 15;242(3):797–802. doi: 10.1042/bj2420797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta E. E., de Meis L. Adenosine 5'-triphosphate-orthophosphate exchange catalyzed by the Ca2+-transport ATPase of brain. Activation by a small transmembrane Ca2+ gradient. J Biol Chem. 1978 Nov 10;253(21):7821–7825. [PubMed] [Google Scholar]
- Ueda T., Chueh S. H., Noel M. W., Gill D. L. Influence of inositol 1,4,5-trisphosphate and guanine nucleotides on intracellular calcium release within the N1E-115 neuronal cell line. J Biol Chem. 1986 Mar 5;261(7):3184–3192. [PubMed] [Google Scholar]
- Willcocks A. L., Cooke A. M., Potter B. V., Nahorski S. R. Stereospecific recognition sites for [3H]inositol(1,4,5)-triphosphate in particulate preparations of rat cerebellum. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1071–1078. doi: 10.1016/0006-291x(87)90756-x. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Colvin J. S., Snyder S. H. Inositol trisphosphate receptor localization in brain: variable stoichiometry with protein kinase C. Nature. 1987 Jan 8;325(7000):159–161. doi: 10.1038/325159a0. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Supattapone S., Wilson V. S., Snyder S. H. Characterization of inositol trisphosphate receptor binding in brain. Regulation by pH and calcium. J Biol Chem. 1987 Sep 5;262(25):12132–12136. [PubMed] [Google Scholar]


