Abstract
Aim: This meta-analysis investigates the association between testosterone replacement therapy [TRT] and carotid artery atherosclerosis. Methods: 3 databases were searched for studies up to June 2023 per the PRISMA guidelines. The eligibility criteria comprised RCTs and observational studies involving hypogonadal males receiving exogenous testosterone, in which CIMT was assessed. CAA was the primary outcome, whereas secondary outcomes included HDL, LDL, CRP, total cholesterol and total testosterone. The statistical analysis was performed using Review Manager. Results: Statistical analysis revealed no association between TRT and assessed outcomes. There was a significant increase in total testosterone levels, depicting indirect anti-atherosclerotic effects of TRT. Conclusion: Meta-analysis shows no relation between TRT and CIMT or other markers, allowing its safe usage for hypogonadal males.
Keywords: : atherosclerosis, carotid artery, carotid intima media thickness, exogenous testosterone, hormone replacement therapy, hypogonadal males, inflammatory markers, lipid markers, meta-analysis, testosterone replacement therapy
Plain language summary
Article highlights.
Introduction
Cardiovascular diseases, including atherosclerosis, are major global health concerns, with carotid artery atherosclerosis (CAA) being a critical risk factor for ischemic stroke.
Testosterone replacement therapy (TRT) is administered to individuals with low testosterone levels (hypogonadism), aiming to restore hormonal balance and potentially improve various aspects of health, including mood, energy levels and sexual function.
TRT has been associated with both potential benefits, such as improved lipid profiles and reduced inflammatory markers and concerns, including adverse cardiovascular events.
Conducting a comprehensive meta-analysis focusing on the relationship between TRT and CAA, particularly assessing carotid intima-media thickness (CIMT), is crucial for understanding TRT's impact on cardiovascular health and guiding clinical decision-making for individuals with hypogonadism.
Methods
Meta-analysis was conducted adhering to PRISMA guidelines, utilizing PubMed, Cochrane and MEDLINE databases up to June 2023.
Selection criteria included randomized control trials and observational studies with at least 16 weeks follow-up, focusing on males with hypogonadism comparing TRT with Placebo, assessing CIMT as a marker of carotid atherosclerosis.
Statistical analysis was performed using Review Manager 5.3, employing a random-effects model, subgroup analysis and sensitivity analysis to evaluate heterogeneity and potential biases.
Results
The screening of 844 articles resulted in the selection of four studies comprising 184 participants, assessing the effects of testosterone replacement therapy (TRT) on atherosclerotic markers in hypogonadal males.
No significant relationship was found between TRT and carotid intimal media thickness (CIMT), HDL, LDL, CRP, or total cholesterol. However, TRT significantly increased total testosterone levels.
Subgroup analysis showed insignificant differences in outcomes between randomized controlled trials (RCTs) and observational studies, with overall minimal safety risks associated with TRT observed across atherosclerotic markers.
Discussion
Study involving 184 hypogonadal males found no significant association between CIMT and TRT, indicating safe usage of TRT without major side effects on carotid artery health.
TRT showed no significant impact on HDL, LDL, CRP, or total cholesterol levels, but significantly increased total testosterone levels, potentially benefiting hypogonadal patients.
Despite limitations such as small sample sizes and variations in TRT dosages and patient comorbidities, the study's comprehensive analysis enhances understanding of TRT's effects on atherosclerosis risk markers.
Conclusion
TRT in hypogonadal males is not associated with progression of carotid artery atherosclerosis (CAA) but increases total testosterone levels, suggesting potential anti-atherosclerotic effects. This indicates its safety and potential benefits in hypogonadism management. Further research is required for a comprehensive risk-benefit assessment.
1. Background
Cardiovascular diseases, particularly atherosclerosis, remain a noteworthy worldwide health concern, accounting for a considerable portion of morbidity and mortality [1]. Carotid artery atherosclerosis [CAA] involves the narrowing and hardening of the carotid arteries, which supply oxygen-rich blood to the brain [2]. This condition is a critical risk factor for ischemic stroke, a potentially life-threatening event [3] and accounts for 25.4% of men and 26.4% of women suffering worldwide [4]. Carotid Intima-Media Thickness [CIMT] is the gold standard for assessing the incidence and progression of CAA.
Testosterone replacement therapy [TRT] involves the administration of exogenous testosterone to individuals with low testosterone levels, often referred to as hypogonadism [5,6], and therefore, has emerged as a potential intervention to manage certain medical conditions, including hypogonadism [5].
Hypogonadism is a clinical condition characterized by insufficient testosterone production [7]. It can occur due to various reasons, including age-related decline, genetic factors and certain medical conditions affecting the testes or pituitary gland [8]. The symptoms of hypogonadism can vary widely and may include reduced libido, erectile dysfunction, fatigue, mood changes and decreased muscle mass [7,8]. When diagnosed with hypogonadism and after considering other underlying medical conditions, TRT is prescribed to raise testosterone levels to the normal range [5,7].
TRT exerts its effects through various mechanisms, primarily by increasing testosterone levels in the body [6]. Testosterone plays a crucial role in maintaining the development and function of male reproductive tissues [5], but it also extends beyond reproduction. It impacts bone density, muscle mass, fat distribution, mood regulation and cognitive function [9–13]. Consequently, TRT aims to restore hormonal balance, potentially improving mood, energy levels, sexual function and overall quality of life in affected individuals.
However, there have been reservations about the mainstream use of TRT due to its potential side effects too. In recent times, FDA shut down some TRT programs with references that it has been shown to decrease male fertility, increase hematocrit and induce hepatic derangements, edema [sodium and water retention], gynecomastia, sleep apnea and hypercalcemia [14]. However, the data supporting whether TRT has adverse cardiovascular effects is inconclusive.
Over the years, there has been a growing interest in investigating whether TRT could substantially impact carotid atherosclerosis. Some studies have suggested potential cardiovascular benefits, such as improved lipid profiles, reduced insulin resistance and decreased inflammatory markers [12,15]. Data on indirect markers such as lipids has been incorporated as a secondary outcome resulting from the initial search strategy. The authors recognize the existence of numerous larger studies focused on these indirect markers; however, a comprehensive analysis and discussion of these studies are considered beyond the intended scope of the article. However, concerns have also been raised regarding the carotid-specific risks associated with TRT, including an increased risk of strokes and other adverse cardiovascular events [16]. Hence, the cardiovascular effects of TRT, particularly carotid atherosclerosis, warrant a comprehensive evaluation to guide clinical decision-making.
Several meta-analyses have previously explored the effects of TRT on different aspects of health, including cardiovascular outcomes [17,18]. These analyses have provided valuable insights into TRT's potential benefits and risks, but results have not always been consistent and some studies have reported conflicting findings. While some have shown decrease in cardiovascular biomarkers with the use of TRT [19,20], some have established a negative association of CVS outcomes and TRT using observational studies [21–23]. While others have found no such association [24]. This demands a study which statistically evaluates multiple reports and draw coherent conclusions. Furthermore, no such studies directly measure the risk of developing carotid artery atherosclerosis using the clinical marker of CIMT.
Therefore, conducting a comprehensive meta-analysis focusing specifically on the relationship between TRT and CAA regarding atherosclerotic risk markers, particularly CIMT, becomes imperative. This meta-analysis aims to synthesize and evaluate existing research on the subject, shedding light on the association between TRT and carotid atherosclerosis and whether TRT could potentially contribute to the regression or stabilization of carotid atherosclerosis through its impact on cardiovascular risk factors, inflammation and lipid metabolism.
Understanding the potential effects of TRT on cardiovascular health is essential for healthcare practitioners to make informed decisions when considering TRT for patients with hypogonadism. The findings of this study may pave the way for a deeper understanding of TRT's role in managing cardiovascular risk factors and, in turn, contribute to the advancement of patient care and management strategies for individuals with hypogonadism.
2. Methods
2.1. Data sources & search strategy
This meta-analysis was conducted in conformity with PRISMA guidelines [25]. The articles were searched through PubMed, Cochrane and MEDLINE databases. The search strategy included articles up to June 2023. A detailed search strategy is given in Supplementary Table S1.
2.2. Study selection & eligibility criteria
Articles selected after the systematic search were moved to EndNote Reference Library Software. All the duplicates were removed while the remaining research articles were first screened based on title and abstract. After narrowing down the studies through initial screening, further screening occurred by going through the full text to assess the relevance of the studies to the topic of our meta-analysis. Articles were screened independently by TWO reviewers [A.I. and S.H.H.]. Any discrepancies were resolved by discussion till consensus.
The inclusion criterion for the studies in our meta-analysis was based on randomized control trials and observational studies with follow-up for at least 16 weeks, a population of males with hypogonadism, studies in which TRT was compared with Placebo, studies in which change in CIMT was reported as it is the most relevant marker indicating carotid atherosclerotic changes. Whereas all the studies in which CIMT was not mentioned as an outcome to assess for TRT, all case series, case reports and articles in languages other than English were excluded.
2.3. Outcomes of interest, data extraction, & quality assessment
The primary outcome that was assessed in our meta-analysis was CAA, whereas the secondary outcomes included generalized atherosclerosis, fatigue, endothelial dysfunction, vascular pathologies, stroke, mortality and syncope,
The important and relevant data, such as baseline demographics, outcome data and safety from the selected studies, were extracted into an Excel spreadsheet. After that, the Cochrane Risk of Bias Tool [26] and the Newcastle and Ottawa Scale were used to assess the quality of the studies selected. Two reviewers [A.I. and S.H.H.] independently conducted quality assessment and the discrepancies were resolved via discussion.
2.4. Statistical analysis
Review Manager [RevMan version 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014] was used for all the statistical analyses. A random-effects model was selected to account for the expected high heterogeneity in the study designs and certain outcomes of our analysis. A 95% confidence interval mean difference assessed the continuous outcomes, subsequently assigning the study weights using an inverse variance method. A p-value of <0.05 was considered significant.
Since there were three RCTs and one observational study, a subgroup analysis was also conducted to assess the effect of any sub-group differences on the data. The χ 2 test was used to test for subgroup differences. Furthermore, we used the Higgins I 2 statistic to evaluate the statistical heterogeneity and considered it significant when it was >50%. We also conducted the sensitivity analysis by employing the leave-one-out analysis to identify the trial-causing heterogeneity of >50%. We could not evaluate any publication bias visually in funnel plots since the number of studies used in this meta-analysis was less than 10.
3. Results
3.1. Study characteristics
A total of 844 relevant articles were initially identified using the pre-defined search criteria by a primary computerized literature search from the Cochrane, Pubmed and Medline databases. Out of 844 studies, 379 that were duplicates were removed. 465 records went through initial screening, out of which 416 articles were excluded and 49 studies were selected for full-text screening. After the full-text screening, 45 studies were removed due to either not meeting our direct outcome or patient inclusion criteria. We selected four studies to be included in the quantitative analysis, as shown in Supplementary Figure S1. All the articles were written in English and met our pre-defined inclusion and outcome criteria.
The four studies included 184 men, 118 of whom received the TRT treatment and were taken as a treatment group, whereas 66 were given the placebo. Aversa et al. [27], Dogan et al. [28], Mathur et al. [29] and assessed the clinical outcomes at 24 months, 3–6 months and 12 months, respectively. In contrast, Ferreira et al. [24], compared the outcomes of the patients newly started on TRT and those being treated for >1 year.
3.2. Participants
We combined results from four studies that had 184 participants in total. The mean age of the participants in our research was mainly around >30 years and <60 years [mean: 50.125 years]. We included three randomized controlled trials and one observational study for our meta-analysis. One RCT used Nebido [29], another [27], used Testosterone Undecanoate [TU] and the third one [28] was given Androgen replacement therapy according to the Algorithm of Testosterone Therapy in Adult Men with Androgen Deficiency Syndromes. All four studies included hypogonadal males at baseline, but two of them [27,29] also included other health problems like metabolic syndrome and angina pectoris at baseline, respectively.
In these studies, patients were given different TRT dosages and assessed accordingly. Patients from Mathur et al. were given a 1000 mg dose, patients from Dogan et al. were given a 5 mg dose, whereas patients from Aversa et al. were given a dosage of 1000 mg/12 weeks from week 6 to week 24. Ferreira et al. was an observational study; thus, no TRT was given to those patients.
The baseline characteristics of our test subjects have been written in Supplementary Table S2. We analyzed the combined data from all of these studies to find a relation between TRT and our outcomes.
3.3. Intervention & comparison
All three RCTS compared TRT with control placebos, whereas one observational study [24] compared individuals who had been on TRT for >1 year and the controls who had just been diagnosed with hypogonadism and started TRT.
3.4. Outcomes & meta-analysis
Our meta-analysis included 4 studies in total, out of which three [Aversa A, [27] Dogan BA [28] and Mathur A [29]] were randomized control trials [RCTs] and one [Ferreira MDA [24]] was an observational study. Our research aimed to study the effects of Testosterone replacement therapy [TRT] on atherosclerotic markers in hypogonadal males. Our study's primary outcome was to determine if there is a significant relationship between TRT and carotid intimal media thickness [CIMT], HDL, LDL, CRP, total cholesterol and total testosterone.
3.5. Carotid intimal medial thickness
We studied carotid intimal media thickness as our primary outcome. We combined data from all four relevant studies, amounting to 184 participants. Our analysis indicated no significant relation between TRT and CIMT, indicating that it does not play a role in exacerbating or initiating carotid artery disease leading to CAA. [95% CI, p-value = 0.57, Heterogeneity: 97%, Mean Difference: 0.08 [-0.35,0.19]] (Figure 1). Initially, when we combined the data from all 4 studies, we found the heterogeneity to be significant among them. However, the heterogeneity decreased significantly when the studies underwent sensitivity analysis, and we eliminated Mathur [3]. [95% CI, p-value = 0.21, heterogeneity: 0%, mean difference: 0.04 [-0.02, 0.10]] [Supplementary Figure S5]. We did not find a decrease in heterogeneity when any other studies were removed. The heterogeneity was significant because of Mathur [29] because this study only included a small sample size [13 patients] and individuals above 60 years who were angina pectoris patients.
Figure 1.
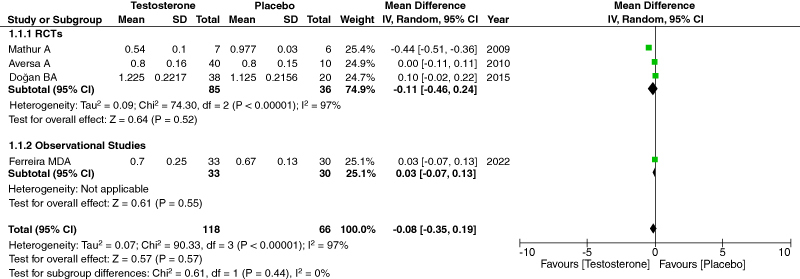
Forest Plot of CIMT.
3.6. HDL
Changes in HDL levels were also studied as an outcome of TRT therapy. We combined data from three relevant pieces of research, amounting to 171 participants. Our analysis indicated low heterogeneity and no significant correlation between TRT and HDL. Thus, its low or high level has practically no effect on TRT. [95% CI, p-value = 0.19, heterogeneity: 30%, mean difference: 2.19 [-1.09, 5.47]] (Figure 2).
Figure 2.
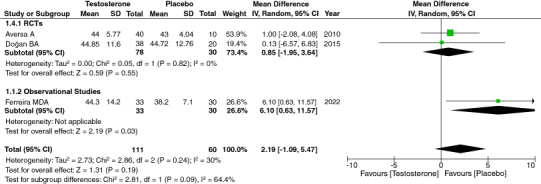
Forest Plot of HDL.
3.7. LDL
We analyzed increased LDL levels as another outcome of TRT therapy. We evaluated LDL increase as an outcome of TRT and found low heterogeneity among the studies. [95% CI, p-value = 0.06, heterogeneity: 0%, mean difference: -12.94 [-26.50, 0.62]]. (Figure 3) Our analysis indicated no significant relationship between increased LDL levels and TRT.
Figure 3.
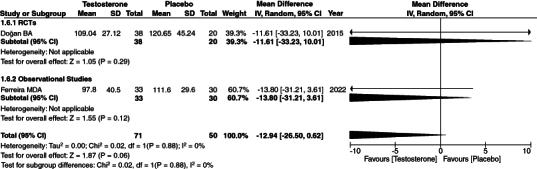
Forest Plot of LDL.
3.8. CRP
CRP is an acute marker of inflammation. We evaluated the effect of TRT therapy on CRP as an outcome of our study. To evaluate the effect of TRT on CRP levels, we combined relevant data from four studies, amounting to 181 participants. Our analysis showed low heterogeneity among studies and no effect of TRT on CRP levels. [95% CI, p-value = 0.45, heterogeneity: 0%, mean difference: -0.01 [-0.07,0.04]] (Figure 4).
Figure 4.
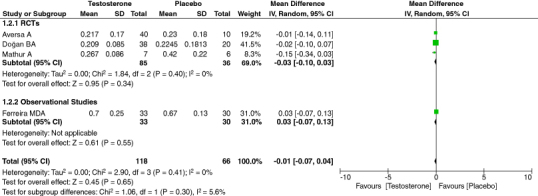
Forest plot of CRP.
3.9. Total cholesterol
Our meta-analysis conclusively evaluated the relationship between increased total cholesterol levels and TRT. We first combined three studies comprising 121 patients and found the results to have high heterogeneity [95% CI, p-value = 0.78, heterogeneity: 91% and mean difference: 6.0[-36.02, 48.02]] (Figure 5). In order to remove the heterogeneity, we performed a sensitivity analysis. When we removed Aversa [27], our heterogeneity was insignificant. However, such was not the case when any other study was removed. [95% CI, p-value = 0.16, heterogeneity: 0%, mean difference: -12.72 [-30.40,4.960] (Supplementary Figure S6). The reason the heterogeneity was high due to Aversa [27], is that the patient population had metabolic syndrome and Type 2 diabetes mellitus and were followed up within the course of 24 months.
Figure 5.
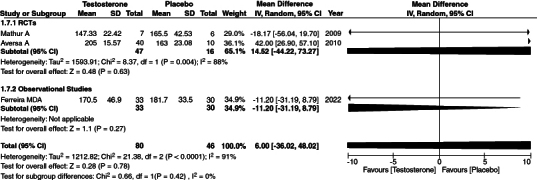
Forest plot of total cholesterol.
3.10. Total testosterone
In our meta-analysis, we evaluated the effect of TRT on total testosterone. There was a significant relation between increased total testosterone levels with TRT. We first combined all four of the studies with a total of 184 participants. We analyzed the data and found results with a high heterogeneity. [95% CI, p-value = 0.14, heterogeneity: 99%, mean difference: 5.92 [-1.87, 13.70]] (Figure 6).
Figure 6.
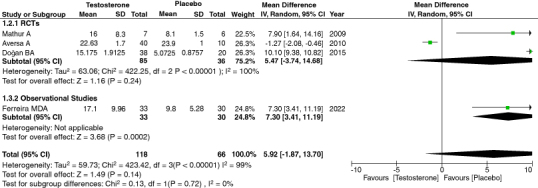
Forest plot of total testosterone.
A sensitivity analysis was performed in order to remove the heterogeneity. The heterogeneity was found to be insignificant when Aversa et al. was removed [95% CI, p-value = <0.00001, heterogeneity: 15%, mean difference: 9.65 [8.19, 11.10]] (Supplementary Figure S7). Our analysis indicated a significant p-value, directly correlating high testosterone levels in patients treated with TRT. The reason the heterogeneity was high due to Aversa [27] is that the patient population had metabolic syndrome and Type 2 diabetes mellitus and were followed up within the course of 24 months.
3.11. Subgroup analysis
Since the study designs were different in nature, we divided them into subgroups consisting of 3 RCTs and one observational study. It should be noted that the subgroup analysis had no effect on the results, and the subgroup differences in the outcomes were insignificant. The subgroup differences for CIMT are p = 0.82, HDL is p = 0.09, LDL is p = 0.88, CRP is p = 0.30, total cholesterol is p = 0.75 and total testosterone is p = 0.17. These differences are insignificant and thus were not found to modify the TRT results on our data's atherosclerotic markers.
3.12. Safety
The overall pool of patients found that the risk of testosterone replacement therapy for atherosclerotic markers is minimal to none. We found out that there was minimal risk of CIMT with TRT [95% CI, p-value = 0.21, heterogeneity: 0%, mean difference: 0.04 [-0.02, 0.10]], as well as other atherosclerotic markers such as HDL [95% CI, p-value = 0.19, heterogeneity: 30%, mean difference: 2.19 [-1.09, 5.47]], LDL [95% CI, p-value = 0.06, Heterogeneity: 0%, Mean difference: -12.94 [-26.50, 0.62]], CRP [95% CI, p-value = 0.45, heterogeneity: 0%, mean difference: -0.01 [-0.07,0.04]], and total cholesterol [95% CI, p-value = 0.16, heterogeneity: 0%, mean difference: -12.72 [-30.40,4.960]. In fact, we found total testosterone to be increased [95% CI, p-value = <0.00001, heterogeneity: 15%, mean difference: 9.65 [8.19, 11.10]] because of TRT and thus contributing to anti-atherosclerotic effects of testosterone on the body.
3.13. Risk bias
We carried out Cochrane's risk of bias assessment on the trials; they generally had a low risk of bias. The studies that we used for our meta-analysis were well-reported. While there were some unclear risks of bias in Mathur et al. and Aversa et al., and some high risks of bias in Dogan et al., such as the unclear random sequence generation and the allocation concealment, all the studies generally had a low risk of bias in all the other aspects (Figures 2, 3).
We used the New Castle Ottawa Quality Assessment Scale to assess risk bias for our observational study. (Figure 4) We found that the case definition was adequate with independent validation and was representative of the community in which the study was conducted. The control group was selected from the same database as the exposed group. The definition of the control group was having no history of the disease, and the comparability of the study was adjusted for age and duration of receiving TRT. It was ascertained that the exposed group received the treatment, and the same methods were used at the same rate to ascertain the exposed and control groups.
4. Discussion
The primary objective of this research, involving 184 hypogonadal males, was to evaluate the relationship between Carotid Artery Intima Thickness [CIMT] and Testosterone Replacement Therapy [TRT] and their effects on overall atherosclerosis risk. CIMT is considered a relevant marker for assessing carotid atherosclerotic changes and understanding its association with TRT is critical for CVS health. Our study investigated this relationship by analyzing data from a collection of relevant studies, revealing no significant association between CIMT and TRT, paving the way for safe usage by confirming no major side effects on carotid artery health. Moreover, our study evaluated parameters such as HDL, LDL, CRP and cholesterol levels alongside CIMT, which has not been done in previous meta-analyses.
These findings elicit TRT's impact on CAA and highlight other factors influencing atherosclerotic progression. Several previous studies have explored the effects of testosterone on atherosclerosis. A review published in the Journal of the American Heart Association [JAMA] experimentally showed how testosterone inhibits atherosclerotic plaque formation – which might indicate a potential protective effect of testosterone in atherosclerotic patients [19]. Data also expresses a negative association between testosterone and CVS risk [21]. A study published in Journal of Clinical Endocrinology and Metabolism [JCEM] by Mohler et al., experimentally showed reduction in cardiovascular biomarkers in hypogonadal patients on who testosterone gel was used for 12 months to keep the hormone levels in the physiological range [20]. The study showed significant reduction in serum cholesterol, HDLs, LDLs from baseline to the end of 12th month. Moreover, a small but notable reduction in insulin resistance was also observed as baseline insulin levels decreased in these patients. Findings by Mohler et al., are in accordance with ours where our statistical findings show decrease in CIMT, a strong marker for CAA, in patients undergoing TRT.
Similarly, Corona et al. [22], Giovanni et al. [23] and Oskui et al. [30]. all investigated the impact of TRT on CVS outcomes and reported a significant reduction in CVS events. In comparison, a recent study published in the Lancet reported no impact of TRT on CVS outcomes [31]. Furthermore, trials included in our meta-analysis by Mathur et al., Dogan et al. and Aversa et al. reported a significant association between TRT and a reduction in CIMT [27–29]. These variations in results across different meta-analyses emphasize the complexities involved in assessing the effects of TRT on CVS health and underscore the importance of considering specific outcomes in future studies.
Our analysis showed no indication between CIMT and TRT, suggesting TRT has no effect on the incidence or progression of CAA. This finding makes it evident that the patient population can safely use TRT without any side effects on carotid artery health. Similar results have also been reported in a study, further supporting our findings [24]. However, the association of TRT with CAA is a complex one, especially the long-term effects of the treatment on hypogonadal males. This is further supported by an on-going clinical trial which is set to evaluate the long-term effects of testosterone replacement therapy on the cardiovascular system of men with hypogonadism [TRAVERSE] study. This study will undertake 6000 subjects and will evaluate the CVS outcomes for next 5 years. After the publication of the findings, more light will be shed on this complex phenomenon.
We also found no significant association between TRT and high-density lipoprotein [HDL] levels or low-density lipoprotein [LDL] levels, where the mentioned parameters were evaluated as an outcome of the study. As per our findings, the potential risk of developing dyslipidemia and related disorders with the clinical use of TRT can be ruled out. Conditions such as heart attack, stroke, CAA, DMT2 and peripheral polyneuropathy are all linked with low HDL or high LDL levels in the body [32–34]. Our results contrast with a study by Zitzmann et al., which found that TRT can lower HDL-cholesterol levels, raising concerns about TRT's overall negative metabolic impact [35]. In contrast, a study by Debing et al. seconded our findings by deducing that TRT might reduce total cholesterol levels but may not significantly affect HDL or LDL cholesterol levels [36]. Another key finding from our analysis showed that there is no link between TRT and total cholesterol levels. This association is in stark contrast with many reports in literature. For example, a meta-analysis published by Kim et al. showed a negative association between serum cholesterol and testosterone [37].
All these findings supported by published literature indicates how the use of TRT may prove beneficial in males with testosterone deficiency, especially those suffering from hypogonadism. Recently guidelines by Italian Society of Andrology and Sexual Medicine [SIAMS] as well as the Italian Society of Endocrinology [SIE] published in 2022, recommends the use of TRT to all the symptomatic patients of hypogonadism after exclusion of other possible contraindications have been met [38]. Moreover, Society of Endocrinology also suggests the use of testosterone transdermal gel or testosterone intramuscular injections for containment of hyperlipidemic effects of congenital and acquired hypogonadal conditions including but not limited to adrenal hypoplasia congenita, Prader-Willi, Bardet-Biedl syndrome, Klinefelter syndrome and cryptorchidism [39]. Hence, our findings are coherent with these guidelines as the result show the use of TRT has no role in the progression of these diseases and, therefore, can be safely used by hypogonadal males.
TRT does not lead to a significant increase in C-reactive protein [CRP] levels, indicating that TRT may not substantially affect inflammation, which may lead to CAA. These findings negate the claims that using TRT might lead to a pro-atherosclerotic state. Therefore, according to our data, TRT can be safely used in hypogonadal patients who are obese, have DMT2, or smoke – conditions that are all associated with a high CRP level in the body without further exacerbating the pro-inflammatory state. A study by Skogastierna et al. also concedes with our findings that TRT has no effect on CRP levels [40].
Nonetheless, it is important to note that TRT had a significant effect on increasing total testosterone levels. Hence, patients, especially those with hypogonadism, can greatly benefit from the clinical use of TRT. Recent statistics indicate that by the year 2030, approximately 16.47% of the global population, equating to an estimated 1.4 billion individuals, will be aged 60 years or older [41]. Moreover, projections suggest that 578 million people, constituting approximately 6.8% of the total population, will be afflicted with Type 2 diabetes mellitus [DMT2] by the same year [42]. Given the escalating prevalence of DMT2 and the aging demographic, testosterone replacement therapy [TRT] emerges as a potentially significant intervention. Research findings reveal that 56.8% of individuals diagnosed with hypogonadism concurrently exhibit dyslipidemia [43]. Notably, among those with DMT2 as an additional comorbidity, the prevalence of dyslipidemia stands at 47% [44]. Furthermore, testosterone also has profound anti-atherosclerotic effects; normal and elevated testosterone levels have been found to lower the risk of stroke [45]. Whereas low levels of testosterone correspond to an increased incidence of strokes in males, showcasing the protective effect of the hormone on carotid artery health [46]. Some cited features of testosterone to increase bone density improve metabolic markers, including better fat distribution, muscle strength and mass, improved sex drive and sperm production [47–49]. Warranting the use of TRT in clinical practice effectively and holistically while simultaneously eradicating the myths and hesitations surrounding the use of testosterone in hypogonadal and elderly patients.
While our study did highlight a few key points regarding the use and manifestation of TRT in important serum atherosclerotic markers, certain limitations should be addressed. One barrier that hindered the statistical power of our findings was the small sample size in all the available studies. Moreover, the studies we used for meta-analysis were of subcategories comprised of RCTs and a case-control study, which might have produced heterogeneity in the calculated data. However, to overcome this, we performed a subgroup analysis. A key discrepancy we observed while performing analysis was variation in the dosages of TRT and follow-up times of patients in each study. Two studies also featured patients suffering from other comorbidities e.g., angina [in Mathur et al.] and DMT2 [in Aversa et al.], which might have alterations in serum biomarkers for CAA risk. To mitigate any discrepancy, sensitivity analysis was performed using the leave-one-out method to ensure the viability of our findings.
Notwithstanding the limitations, our study possesses several strengths that enhance its validity and significance. We used a variety of subcategories from the literature to enhance the inclusivity and comprehensiveness of our study. A key highlight of our study was taking CIMT as a marker for CAA risk – something previous meta-analyses had not done. CIMT is a more nuanced marker for the atherosclerotic state of the carotid arteries, as it has a quantifiable value. Hence, this value was assessed statistically against TRT usage and results were drawn out. In addition to that, important serum markers such as HDL, LDL, CRP and total cholesterol levels were evaluated alongside CIMT to enhance the robustness of the study. An important tool that helped us overcome our shortcomings was performing sensitivity analysis and quality assessment on the included studies to ensure the consistency of the findings and the reliability of our results.
Though the literature on testosterone, TRT usage and overall CAA risk is vast, there are certain gaps in the studies we found while screening. One avenue for future investigations could involve exploring the effects of TRT in eugonadal males. This could provide key insights into the mechanism of TRT across a more diverse population.
5. Conclusion
In essence, our meta-analysis investigated the impact of TRT on CAA in hypogonadal males. The results show no significant association between TRT and CIMT and other markers, including LDL, HDL, CRP and total cholesterol; establishing TRT does not lead to or increase the progression of CAA. However, TRT was associated with increased total testosterone levels, showing its anti-atherosclerotic actions. Our research shows the potential benefits of TRT in patients with hypogonadism, and it is considered safe in terms of atherosclerotic changes and potentially offers protection against it. However, further studies are needed to explore more of its risks and benefits.
Supplementary Material
Supplemental material
Supplemental data for this article can be accessed at https://doi.org/10.1080/20565623.2024.2365125
Author contributions
SH Haider: methodology, investigation, formal analysis, visualization, writing the original manuscript and project administration. A Irfan: methodology, investigation, formal analysis, visualization and project administration. S Mustafa: methodology and writing the original manuscript. T Abid: writing the original manuscript. T Naz: data extraction. M Abbas: writing the original manuscript. A Raza: data extraction.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Jagannathan R, Patel SA, Ali MK, et al. Global updates on CVS disease mortality trends and attribution of traditional risk factors. Curr Diabetes Rep. 2019;19(7):44. doi: 10.1007/s11892-019-1161-2 [DOI] [PubMed] [Google Scholar]; •• Provides global updates on cardiovascular disease mortality trends and the attribution of traditional risk factors, offering essential context for understanding the broader implications of cardiovascular health in the population.
- 2.Martinez E, Martorell J, Riambau VJJoVS. Review of serum biomarkers in carotid atherosclerosis. J Vasc Surg. 2020;71(1):329–341. doi: 10.1016/j.jvs.2019.04.488 [DOI] [PubMed] [Google Scholar]; •• Examines serum biomarkers associated with carotid atherosclerosis, offering insights into potential indicators for disease progression or risk assessment.
- 3.Romero JR, Beiser A, Seshadri S, et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke. 2009;40(5):1590–1596. doi: 10.1161/STROKEAHA.108.535245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prati P, Vanuzzo D, Casaroli M, et al. Prevalence and determinants of carotid atherosclerosis in a general population. Stroke. 1992;23(12):1705–1711. doi: 10.1161/01.STR.23.12.1705 [DOI] [PubMed] [Google Scholar]; •• Investigates the prevalence and determinants of carotid atherosclerosis in a general population, providing foundational knowledge for understanding the epidemiology and risk factors associated with the condition.
- 5.Rastrelli G, Guaraldi F, Reismann Y, et al. Testosterone replacement therapy for sexual symptoms. Sex Med Rev. 2019;7(3):464–475. doi: 10.1016/j.sxmr.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 6.Barbonetti A, D'Andrea S, Francavilla S. Testosterone replacement therapy. Andrology. 2020;8(6):1551–1566. doi: 10.1111/andr.12774 [DOI] [PubMed] [Google Scholar]
- 7.Corona G, Goulis DG, Huhtaniemi I, et al. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: Endorsing organization: European Society of Endocrinology. Andrology. 2020;8(5):970–987. doi: 10.1111/andr.12770 [DOI] [PubMed] [Google Scholar]
- 8.Thirumalai A, Anawalt BDJE, America mcoN. Epidemiology of male hypogonadism. Endocrinol Metab Clin North Am. 2022;51(1):1–27. doi: 10.1016/j.ecl.2021.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]; • Provides a comprehensive overview of the epidemiology of male hypogonadism, which is relevant to understanding the prevalence and incidence of the condition within the population.
- 9.Corona G, Vena W, Pizzocaro A, et al. Testosterone supplementation and bone parameters: a systematic review and meta-analysis study. J Endocrinol Invest. 2022;45(5):911–926. doi: 10.1007/s40618-021-01702-5 [DOI] [PubMed] [Google Scholar]
- 10.Kruse R, Petersson SJ, Christensen LL, et al. Effect of long-term testosterone therapy on molecular regulators of skeletal muscle mass and fibre-type distribution in aging men with subnormal testosterone. Metabolism. 2020;112:154347. doi: 10.1016/j.metabol.2020.154347 [DOI] [PubMed] [Google Scholar]
- 11.Rogne A, Hassel B. Improvement of attention deficit/hyperactivity disorder (ADHD) in three adult men during testosterone treatment: a case series. J Med Case Rep. 2022;16(1):425. doi: 10.1186/s13256-022-03651-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuhara S, Mori J, Nakajima HJCPE. Klinefelter syndrome in an adolescent with severe obesity, insulin resistance, and hyperlipidemia, successfully treated with testosterone replacement therapy. Clin Pediatr Endocrinol. 2021;30(3):127–132. doi: 10.1297/cpe.30.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauger RL, Saelzler UG, Pagadala MS, et al. The role of testosterone, the androgen receptor, and hypothalamic-pituitary–gonadal axis in depression in ageing men. Rev Endocr Metab Disord. 2022;23(6):1259–1273. doi: 10.1007/s11154-022-09767-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metzger SO, Burnett AL. Impact of recent FDA ruling on testosterone replacement therapy (TRT). Translat Androl Urol. 2016;5(6):921–926. doi: 10.21037/tau.2016.09.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheetham TC, An J, Jacobsen SJ, et al. Association of testosterone replacement with CVS outcomes among men with androgen deficiency. JAMA Intern Med. 2017;177(4):491–499. doi: 10.1001/jamainternmed.2016.9546 [DOI] [PubMed] [Google Scholar]; •• Examines the association of testosterone replacement therapy with cardiovascular outcomes among men with androgen deficiency, contributing valuable evidence to the ongoing discourse on the cardiovascular effects of testosterone therapy.
- 16.Ide V, Vanderschueren D, Antonio L. Treatment of men with central hypogonadism: alternatives for testosterone replacement therapy. Inter J Mol Sci. 2020;22(1):21. doi: 10.3390/ijms22010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corona G, Rastrelli G, Maggi MJBp, et al. Diagnosis and treatment of late-onset hypogonadism: systematic review and meta-analysis of TRT outcomes. 2013;27(4):557–579. doi: 10.1016/j.beem.2013.05.002 [DOI] [PubMed] [Google Scholar]; • This systematic review and meta-analysis offer insights into the diagnosis and treatment outcomes of late-onset hypogonadism, providing valuable evidence for the efficacy and safety of testosterone replacement therapy.
- 18.Borst SE, Shuster JJ, Zou B, et al. CVS risks and elevation of serum DHT vary by route of testosterone administration: a systematic review and meta-analysis. BMC Med. 2014;12(1):1–16. doi: 10.1186/s12916-014-0211-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herring MJ, Oskui PM, Hale SL, et al. Testosterone and the CVS system: a comprehensive review of the basic science literature. 2013;2(4):e000271. doi: 10.1161/JAHA.113.000271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohler ER III, Ellenberg SS, Lewis CE, et al. The effect of testosterone on cardiovascular biomarkers in the testosterone trials. J Clin Endocrinol Metabol. 2018;103(2):681–688. doi: 10.1210/jc.2017-02243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu L, Freeman, et al. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108. doi: 10.1186/1741-7015-11-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corona G, Rastrelli G, Di Pasquale G, et al. Testosterone and CVS risk: meta-analysis of interventional studies. J Sexual Med. 2018;15(6):820–838. doi: 10.1016/j.jsxm.2018.04.641 [DOI] [PubMed] [Google Scholar]
- 23.Corona GG, Rastrelli G, Maseroli E, et al. Testosterone replacement therapy and CVS risk: a review. World J Men's Health. 2015;33(3):130–142. doi: 10.5534/wjmh.2015.33.3.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Almeida Ferreira M, Mendonça JA. Long-term testosterone replacement therapy reduces fatigue in men with hypogonadism. Drugs Context. 2022;11:2021-8-12. doi: 10.7573/dic.2021-8-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JP, Altman, et al. Cochrane Bias Methods Group, & Cochrane Statistical Methods Group . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med. 2010;7(10):3495–3503. doi: 10.1111/j.1743-6109.2010.01931.x [DOI] [PubMed] [Google Scholar]; • Investigates the effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome, providing valuable insights into the potential cardiovascular benefits or risks associated with testosterone replacement therapy.
- 28.Doğan BA, Karakılıç E, Tuna MM, et al. Effect of androgen replacement therapy on atherosclerotic risk markers in young-to-middle-aged men with idiopathic hypogonadotropic hypogonadism. Clin Endocrinol (Oxf). 2015;82(3):422–428. doi: 10.1111/cen.12617 [DOI] [PubMed] [Google Scholar]
- 29.Mathur A, Malkin C, Saeed B, et al. Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol. 2009;161(3):443–449. doi: 10.1530/EJE-09-0092. Epub 2009 Jun 19. Erratum in: Eur J Endocrinol. 2009;161(4):653. [DOI] [PubMed] [Google Scholar]
- 30.Oskui PM, French WJ, Herring MJ, et al. Testosterone and the CVS system: a comprehensive review of the clinical literature. J Am Heart Assoc. 2013;2(6):e000272. doi: 10.1161/JAHA.113.000272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudson J, Cruickshank M, Quinton R, et al. Adverse CVS events and mortality in men during testosterone treatment: an individual patient and aggregate data meta-analysis. Lancet Healthy Longev. 2022;3(6):e381–e393. doi: 10.1016/S2666-7568(22)00096-4 [DOI] [PMC free article] [PubMed] [Google Scholar]; • This meta-analysis examines the adverse cardiovascular events and mortality rates among men undergoing testosterone treatment, shedding light on potential risks associated with testosterone replacement therapy in cardiovascular health.
- 32.Kjeldsen EW, Thomassen JQ, Frikke-Schmidt R. HDL cholesterol concentrations and risk of atherosclerotic CVS disease – Insights from randomized clinical trials and human genetics. Biochim et Biophys Acta (BBA). 2022;1867(1):159063. doi: 10.1016/j.bbalip.2021.159063 [DOI] [PubMed] [Google Scholar]
- 33.Yu Y, Hu L, Huang X, et al. BMI modifies the association between serum HDL cholesterol and stroke in a hypertensive population without atrial fibrillation. J Endocrinol Invest. 2021;44:173–181. doi: 10.1007/s40618-020-01288-4 [DOI] [PubMed] [Google Scholar]
- 34.Li D, Zhang QJCGP. Predictive value of TG/HDL-C ratio for diabetic peripheral neuropathy in elderly diabetic patients. Neurol Res. 2020;23(29):3690. [Google Scholar]
- 35.Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Therapeut Clin Risk Manag. 2009;5(3):427–448. doi: 10.2147/TCRM.S3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Debing E, Peeters E, Duquet W, et al. Men with atherosclerotic stenosis of the carotid artery have lower testosterone levels compared with controls. Inter Angiol. 2008;27(2):135–141. [PubMed] [Google Scholar]
- 37.Kim SH, Park JJ, Kim KH, et al. Efficacy of testosterone replacement therapy for treating metabolic disturbances in late-onset hypogonadism: a systematic review and meta-analysis. Inter Urol Nephrol. 2021;53(9):1733–1746. doi: 10.1007/s11255-021-02876-w [DOI] [PubMed] [Google Scholar]
- 38.Isidori AM, Aversa A, Calogero A, et al. Adult-and late-onset male hypogonadism: the clinical practice guidelines of the Italian Society of Andrology and Sexual Medicine (SIAMS) and the Italian Society of Endocrinology (SIE). 2022;45(12):2385–2403. doi: 10.1007/s40618-022-01859-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jayasena CN, Anderson RA, Llahana S, et al. Society for Endocrinology guidelines for testosterone replacement therapy in male hypogonadism. Clin Endocrinol. 2022;96(2):200–219. doi: 10.1111/cen.14633 [DOI] [PubMed] [Google Scholar]
- 40.Zgliczynski S, Ossowski M, Slowinska-Srzednicka J, et al. Effect of testosterone replacement therapy on lipids and lipoproteins in hypogonadal and elderly men. Atherosclerosis. 1996;121(1):35–43. doi: 10.1016/0021-9150(95)05673-4 [DOI] [PubMed] [Google Scholar]
- 41.United Nations, Department of Economic and Social Affairs, Population Division . World Population Ageing 2019: highlights (ST/ESA/SER.A/430). 2019. https://www.un.org/development/desa/dpad/
- 42.International Diabetes Federation . IDF Diabetes Atlas (9th edn). Brussels, Belgium: 2019. Available at: https://www.diabetesatlas.org/en/ [Google Scholar]
- 43.Gianatti EJ, Dupuis P, Hoermann R, et al. Effect of testosterone treatment on constitutional and sexual symptoms in men with Type 2 diabetes in a randomized, placebo-controlled clinical trial. J Clin Endocrinol Metabol. 2014;99(10):3821–3828. doi: 10.1210/jc.2014-1872 [DOI] [PubMed] [Google Scholar]
- 44.Kapoor D, Aldred H, Clark S, et al. Clinical and biochemical assessment of hypogonadism in men with Type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care. 2007;30(4):911–917. doi: 10.2337/dc06-1426 [DOI] [PubMed] [Google Scholar]
- 45.Loo SY, Chen BY, Yu OHY, et al. Testosterone replacement therapy and the risk of stroke in men: a systematic review. Maturitas. 2017;106:31–37. doi: 10.1016/j.maturitas.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 46.Yeap BB, Hyde Z, Almeida OP, et al. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J Clin Endocrinol Metab. 2009;94(7):2353–2359. doi: 10.1210/jc.2008-2416 [DOI] [PubMed] [Google Scholar]
- 47.Weiss RV, Hohl A, Athayde A, et al. Testosterone therapy for women with low sexual desire: a position statement from the Brazilian Society of Endocrinology and Metabolism. Arch Endocrinol Metab. 2019;63:190–198. doi: 10.20945/2359-3997000000152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moriishi T, Ozasa R, Ishimoto T, et al. Osteocalcin is necessary for the alignment of apatite crystallites, but not glucose metabolism, testosterone synthesis, or muscle mass. 2020;16(5):e1008586. doi: 10.1371/journal.pgen.1008586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maggi M, Filippi S, Vignozzi L, et al. Controversial aspects of testosterone in the regulation of sexual function in late-onset hypogonadism. 2020;8(6):1580–1589. doi: 10.1111/andr.12794 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


