Abstract
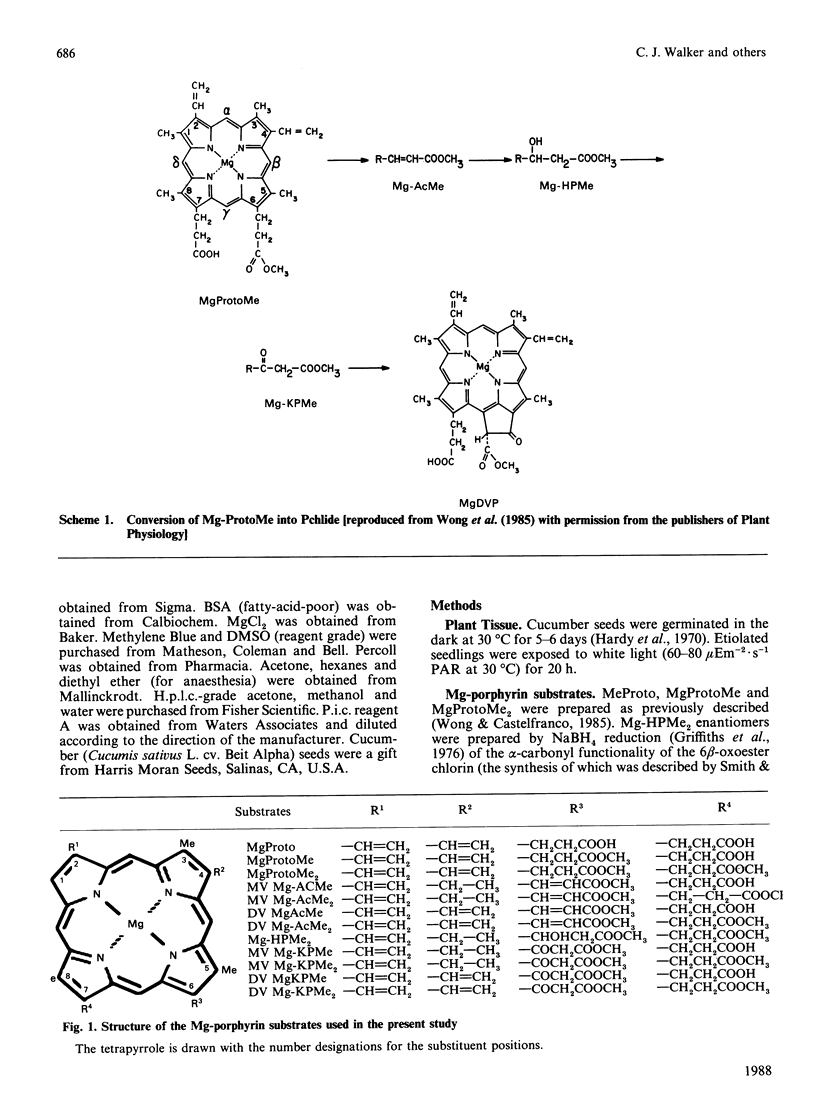
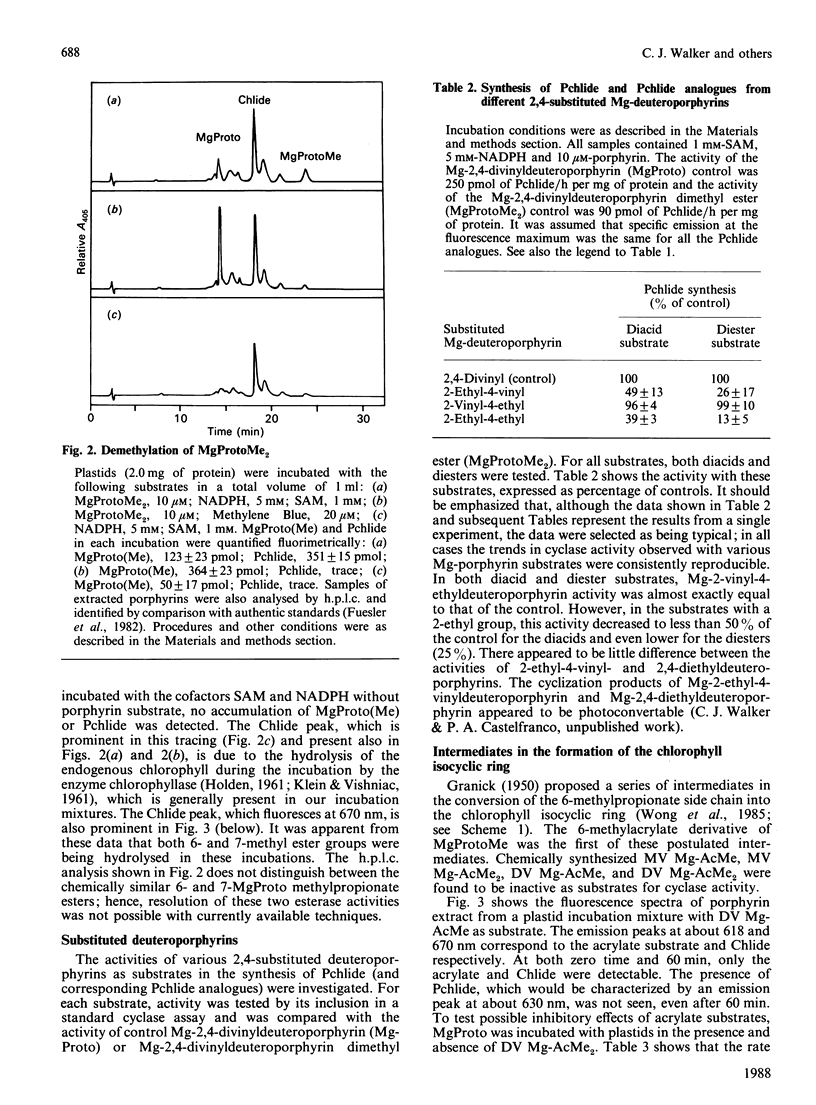
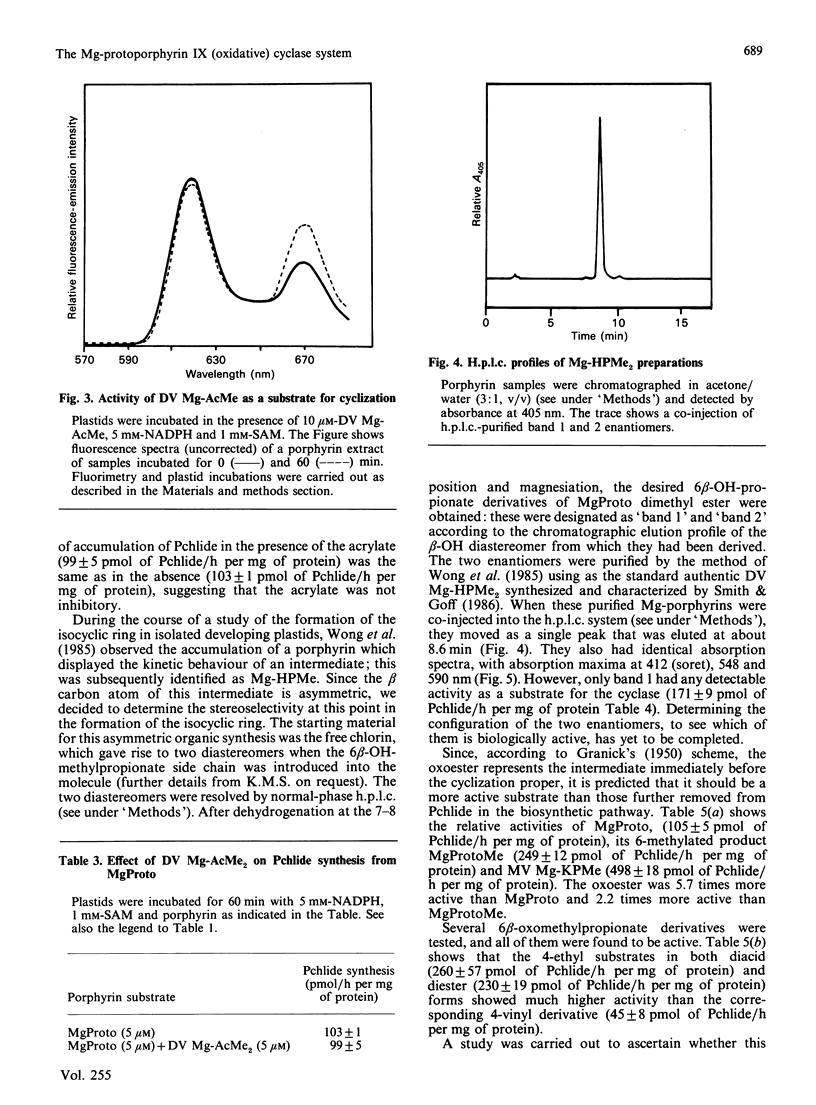
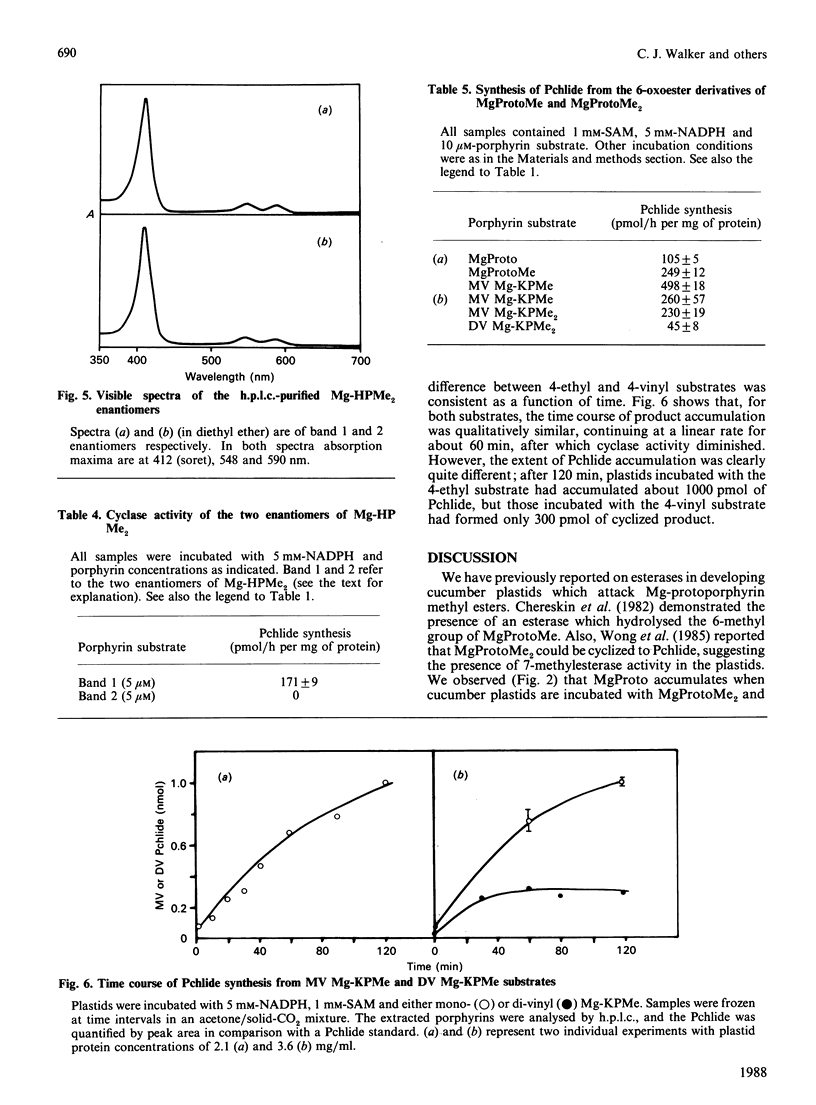
Mg-protoporphyrin IX monomethyl ester cyclase activity was assayed in isolated developing cucumber (Cucumis sativus L. var. Beit Alpha) chloroplasts [Chereskin, Wong & Castelfranco (1982) Plant Physiol. 70, 987-993]. The presence of both 6- and 7-methyl esterase activities was detected, which permitted the use of diester porphyrins in a substrate-specificity study. It was found that: (1) the 6-methyl acrylate derivative of Mg-protoporphyrin monomethyl ester was inactive as a substrate for cyclization; (2) only one of the two enantiomers of 6-beta-hydroxy-Mg-protoporphyrin dimethyl ester had detectable activity as a substrate for the cyclase; (3) the 2-vinyl-4-ethyl-6-beta-oxopropionate derivatives of Mg-protoporphyrin mono- or di-methyl ester were approx. 4 times more active as substrates for cyclization than the corresponding divinyl forms; (4) at the level of Mg-protoporphyrin there was no difference in cyclase activity between the 4-vinyl and 4-ethyl substrates; (5) reduction of the side chain of Mg-protoporphyrin in the 2-position from a vinyl to an ethyl resulted in a partial loss of cyclase activity. This work suggests that the original scheme for cyclization proposed by Granick [(1950) Harvey Lect. 44, 220-245] should now be modified by the omission of the 6-methyl acrylate derivative of Mg-protoporphyrin monomethyl ester and the introduction of stereo-specificity at the level of the hydroxylated intermediate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOETTIGER E. G., FURSHPAN E. The recording of flight movements in insects. Science. 1952 Jul 18;116(3003):60–61. doi: 10.1126/science.116.3003.60. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carey E. E., Rebeiz C. A. Chloroplast Biogenesis 49 : Differences among Angiosperms in the Biosynthesis and Accumulation of Monovinyl and Divinyl Protochlorophyllide during Photoperiodic Greening. Plant Physiol. 1985 Sep;79(1):1–6. doi: 10.1104/pp.79.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey E. E., Tripathy B. C., Rebeiz C. A. Chloroplast biogenesis 51 : modulation of monovinyl and divinyl protochlorophyllide biosynthesis by light and darkness in vitro. Plant Physiol. 1985 Dec;79(4):1059–1063. doi: 10.1104/pp.79.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelfranco P. A., Weinstein J. D., Schwarcz S., Pardo A. D., Wezelman B. E. The Mg insertion step in chlorophyll biosynthesis. Arch Biochem Biophys. 1979 Feb;192(2):592–598. doi: 10.1016/0003-9861(79)90130-9. [DOI] [PubMed] [Google Scholar]
- Chereskin B. M., Wong Y. S., Castelfranco P. A. In Vitro Synthesis of the Chlorophyll Isocyclic Ring : Transformation of Magnesium-Protoporphyrin IX and Magnesium-Protoporphyrin IX Monomethyl Ester into Magnesium-2,4-Divinyl Pheoporphyrin A(5). Plant Physiol. 1982 Oct;70(4):987–993. doi: 10.1104/pp.70.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M. T., Howarth T. T., Jackson A. H., Kenner G. W. Pyrroles and related compounds. 28. Beta-keto-esters in the porphyrin series. J Chem Soc Perkin 1. 1974;4:512–516. doi: 10.1039/p19740000512. [DOI] [PubMed] [Google Scholar]
- Fuesler T. P., Hanamoto C. M., Castelfranco P. A. Separation of Mg-Protoporphyrin IX and Mg-Protoporphyrin IX Monomethyl Ester Synthesized de novo by Developing Cucumber Etioplasts. Plant Physiol. 1982 Feb;69(2):421–423. doi: 10.1104/pp.69.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANICK S. The structural and functional relationships between heme and chlorophyll. Harvey Lect. 1948 1949;Series 44:220–245. [PubMed] [Google Scholar]
- HOLDEN M. The breakdown of chlorophyll by chlorophyllase. Biochem J. 1961 Feb;78:359–364. doi: 10.1042/bj0780359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S. I., Castelfranco P. A., Rebeiz C. A. Effect of the hypocotyl hook on greening in etiolated cucumber cotyledons. Plant Physiol. 1970 Nov;46(5):705–707. doi: 10.1104/pp.46.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN A. O., VISHNIAC W. Activity and partial purification of chlorophyllase in aqueous systems. J Biol Chem. 1961 Sep;236:2544–2547. [PubMed] [Google Scholar]
- Kenner G. W., McCombie S. W., Smith K. M. Pyrroles and related compounds. 30. Cyclisation of porphyrin beta-keto-esters to phaeoporphyrins. J Chem Soc Perkin 1. 1974;4:527–530. doi: 10.1039/p19740000527. [DOI] [PubMed] [Google Scholar]
- Kenner G. W., McCombie S. W., Smith K. M. Pyrroles and related compounds. XXIV. Separation and oxidative degradation of chlorophyll derivatives. J Chem Soc Perkin 1. 1973;21:2517–2523. doi: 10.1039/p19730002517. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Rapoport H. Porphyrin-protein bond of cytochrome c558 from Euglena gracilis. J Am Chem Soc. 1977 May 11;99(10):3479–3485. doi: 10.1021/ja00452a048. [DOI] [PubMed] [Google Scholar]
- Nasrulhaq-Boyce A., Griffiths W. T., Jones O. T. The use of continuous assays to characterize the oxidative cyclase that synthesizes the chlorophyll isocyclic ring. Biochem J. 1987 Apr 1;243(1):23–29. doi: 10.1042/bj2430023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy B. C., Rebeiz C. A. Chloroplast biogenesis. Demonstration of the monovinyl and divinyl monocarboxylic routes of chlorophyll biosynthesis in higher plants. J Biol Chem. 1986 Oct 15;261(29):13556–13564. [PubMed] [Google Scholar]
- Wong Y. S., Castelfranco P. A., Goff D. A., Smith K. M. Intermediates in the formation of the chlorophyll isocyclic ring. Plant Physiol. 1985 Nov;79(3):725–729. doi: 10.1104/pp.79.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. S., Castelfranco P. A. Properties of the Mg-Protoporphyrin IX Monomethyl Ester (Oxidative) Cyclase System. Plant Physiol. 1985 Nov;79(3):730–733. doi: 10.1104/pp.79.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. S., Castelfranco P. A. Resolution and Reconstitution of Mg-Protoporphyrin IX Monomethyl Ester (Oxidative) Cyclase, the Enzyme System Responsible for the Formation of the Chlorophyll Isocyclic Ring. Plant Physiol. 1984 Jul;75(3):658–661. doi: 10.1104/pp.75.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]



