Abstract
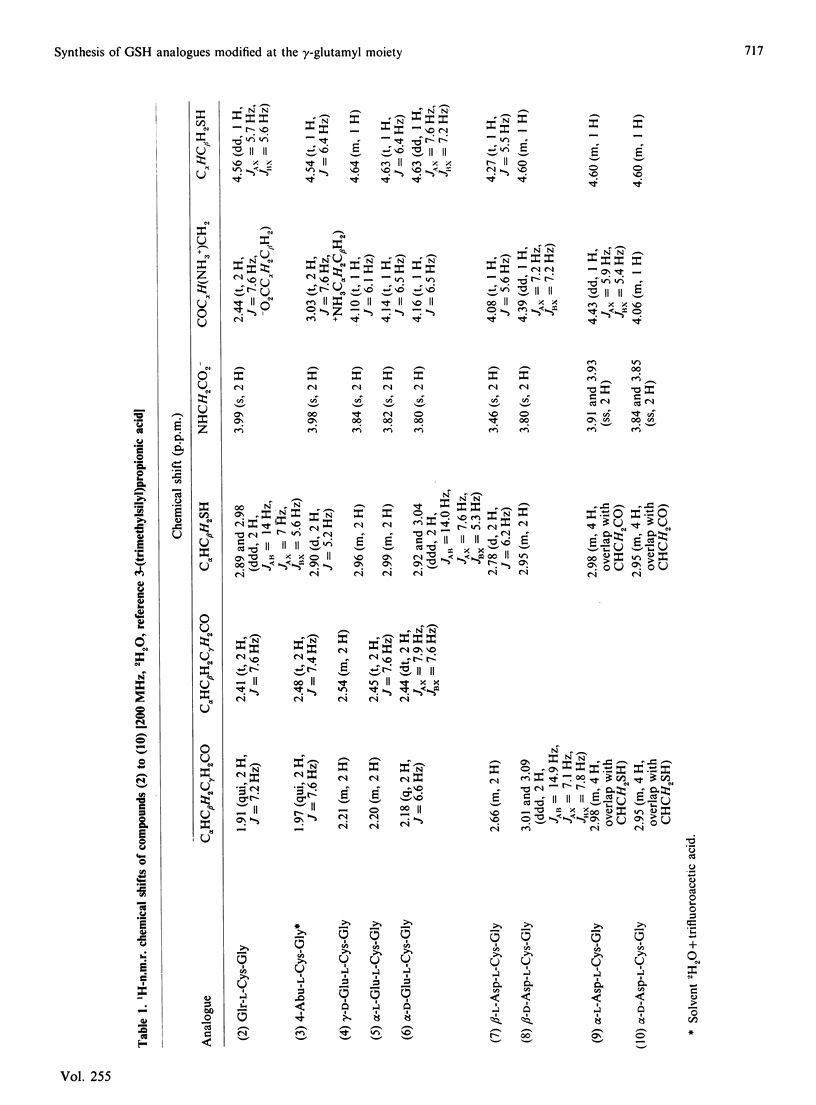
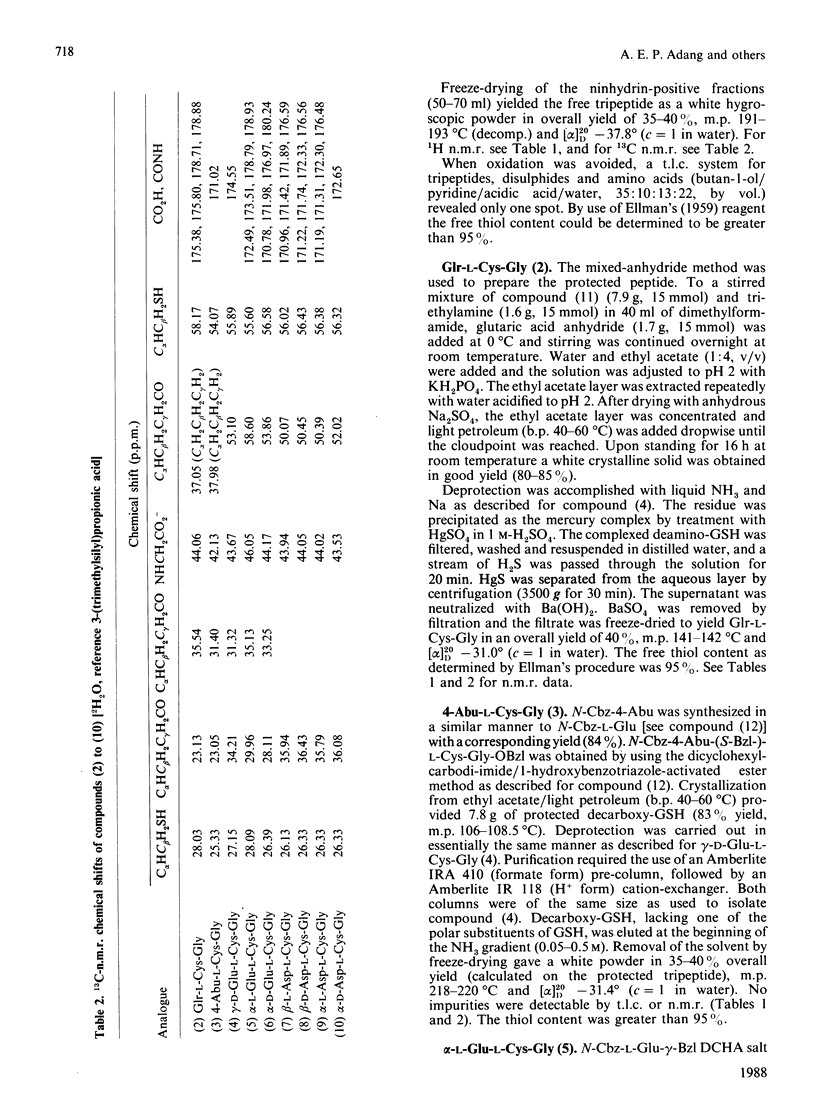
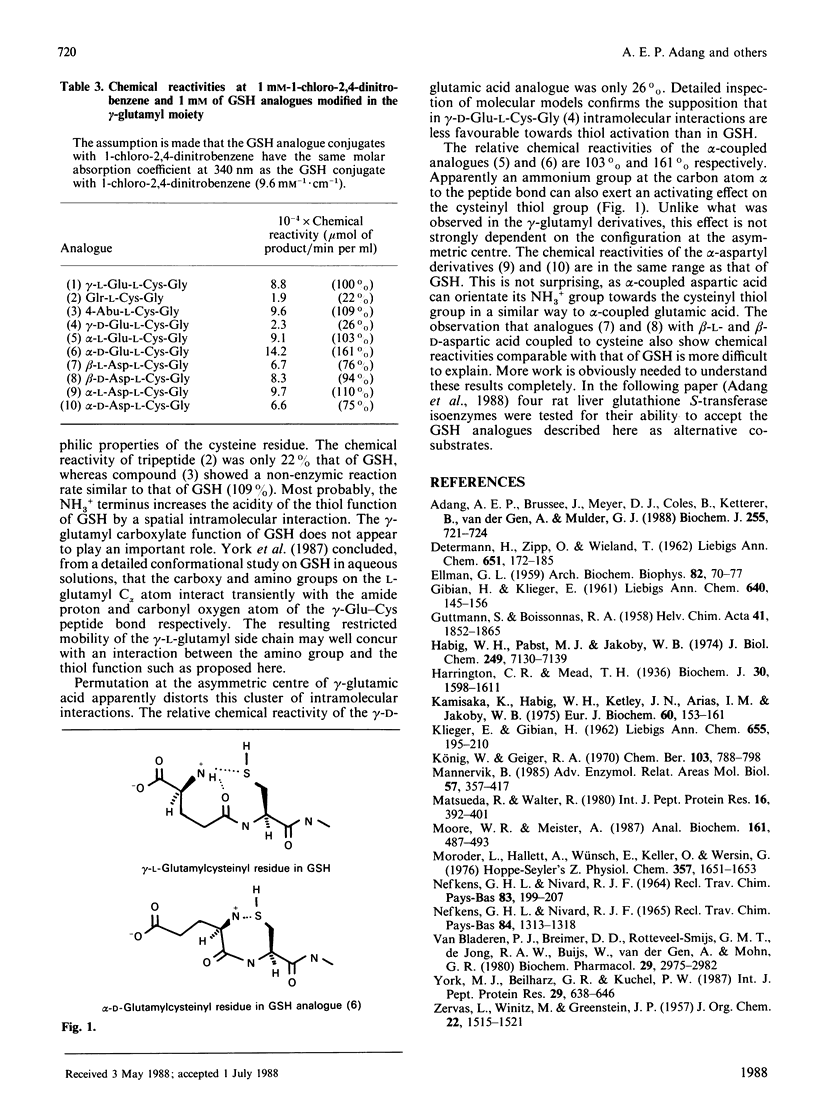
A series of GSH analogues with modifications at the gamma-glutamyl moiety was synthesized and purified by following peptide chemistry methodology. Benzyl, benzyloxycarbonyl and t-butyloxycarbonyl protective groups were used to protect individual amino acid functional groups. The formation of peptide bonds was accomplished through coupling of free amino groups with active esters, generated by reaction of the carboxylate functions with dicyclohexylcarbodi-imide and 1-hydroxybenzotriazole. The protecting groups in the tripeptides were removed in a single step by using Na in liquid NH3. Precautions were taken in order to prevent oxidation of the thiol function in the cysteine residue. Thus GSH analogues containing both L- and D-glutamic acid and L- and D-aspartic acid, coupled to cysteinylglycine through both the alpha- and the omega-carboxylate group, were synthesized. Also, decarboxy-GSH and deamino-GSH, lacking one functional group in the glutamate moiety, were prepared. The spontaneous non-enzyme-catalysed nucleophilic reaction of these GSH analogues with the electrophilic model substrate 1-chloro-2,4-dinitrobenzene showed appreciable rate differences, indicating the importance of intramolecular interactions in determining the nucleophilic reactivity of the thiol function in the cysteine residue. In particular, the free amino group in the gamma-L-glutamic acid residue appears to play a crucial role in activating the thiol group in GSH. In an adjacent paper [Adang, Brussee, Meyer, Coles, Ketterer, van der Gen & Mulder (1988) Biochem. J. 255, 721-724] these results are compared with those obtained in a study on the ability of these GSH analogues to act as a co-substrate in the glutathione S-transferase-catalysed conjugation reaction with 1-chloro-2,4-dinitrobenzene.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adang A. E., Brussee J., Meyer D. J., Coles B., Ketterer B., van der Gen A., Mulder G. J. Substrate specificity of rat liver glutathione S-transferase isoenzymes for a series of glutathione analogues, modified at the gamma-glutamyl moiety. Biochem J. 1988 Oct 15;255(2):721–724. [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Harington C. R., Mead T. H. Synthesis of peptides containing cystine and glutamine, with remarks on their possible bearing on the structure of insulin and a note on the amide nitrogen of insulin. Biochem J. 1936 Sep;30(9):1598–1611. doi: 10.1042/bj0301598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamisaka K., Habig W. H., Ketley J. N., Arias M., Jakoby W. B. Multiple forms of human glutathione S-transferase and their affinity for bilirubin. Eur J Biochem. 1975 Dec 1;60(1):153–161. doi: 10.1111/j.1432-1033.1975.tb20987.x. [DOI] [PubMed] [Google Scholar]
- König W., Geiger R. Eine neue Methode zur Synthese von Peptiden: Aktivierung der Carboxylgruppe mit Dicyclohexycarbodiimid unter Zusatz von 1-Hydroxy-benzotriazolen. Chem Ber. 1970;103(3):788–798. doi: 10.1002/cber.19701030319. [DOI] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Matsueda R., Walter R. 3-nitro-2-pyridinesulfenyl (Npys) group. A novel selective protecting group which can be activated for peptide bond formation. Int J Pept Protein Res. 1980 Nov;16(5):392–401. [PubMed] [Google Scholar]
- Moore W. R., Meister A. Enzymatic synthesis of novel glutathione analogs. Anal Biochem. 1987 Mar;161(2):487–493. doi: 10.1016/0003-2697(87)90478-7. [DOI] [PubMed] [Google Scholar]
- Moroder L., Hallett A., Wünsch E., Keller O., Wersin G. Di-tert.-butyldicarbonat--ein vorteilhaftes Reagenz zur Eingührung der tert.-Butyloxycarbonyl-Schutzgruppe. Hoppe Seylers Z Physiol Chem. 1976 Nov;357(11):1651–1653. [PubMed] [Google Scholar]
- York M. J., Beilharz G. R., Kuchel P. W. Conformation of reduced glutathione in aqueous solution by 1H and 13C n.m.r. Int J Pept Protein Res. 1987 May;29(5):638–646. doi: 10.1111/j.1399-3011.1987.tb02294.x. [DOI] [PubMed] [Google Scholar]
- van Bladeren P. J., Breimer D. D., Rotteveel-Smijs G. M., de Jong R. A., Buijs W., van der Gen A., Mohn G. R. The role of glutathione conjugation in the mutagenicity of 1,2-dibromoethane. Biochem Pharmacol. 1980 Nov 1;29(21):2975–2982. doi: 10.1016/0006-2952(80)90047-7. [DOI] [PubMed] [Google Scholar]


