Abstract
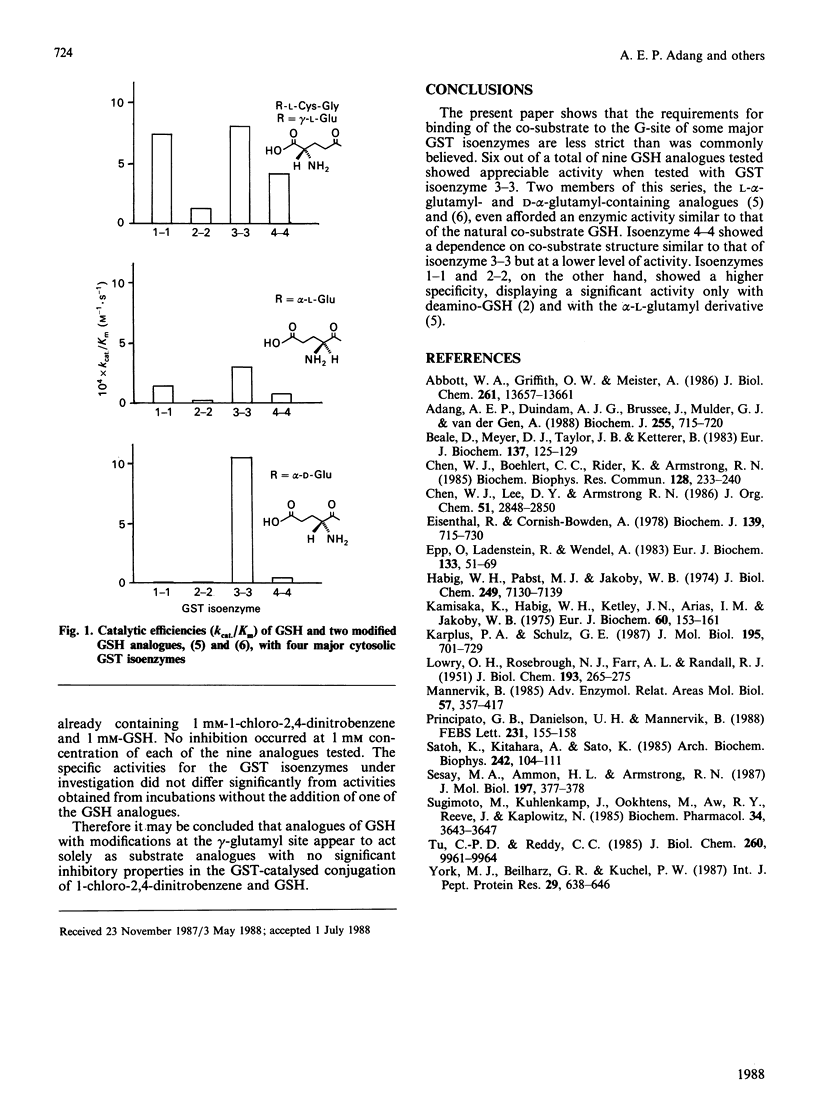
The substrate specificity of purified rat liver glutathione S-transferases (GSTs) for a series of gamma-glutamyl-modified GSH analogues was investigated. GST isoenzyme 3-3 catalysed the conjugation of 1-chloro-2,4-dinitrobenzene with six out of the nine analogues. alpha-L-Glu-L-Cys-Gly and alpha-D-Glu-L-Cys-Gly showed catalytic efficiencies of 40% and 130% that of GSH respectively. The GSH analogue with an alpha-D-glutamyl moiety appeared to be a highly isoenzyme-3-3-specific co-substrate: kcat./Km with GST isoenzyme 4-4 was only about 5% that with GST isoenzyme 3-3, and no enzymic activity was detectable with GST isoenzymes 1-1 and 2-2. GST isoenzyme 4-4 showed some resemblance to GST 3-3: five out of nine co-substrate analogues were accepted by this second isoenzyme of the Mu multigene family. Isoenzymes 1-1 and 2-2, of the Alpha multigene family, accepted only two alternative co-substrates, which indicates that their GSH-binding site is much more specific.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott W. A., Griffith O. W., Meister A. Gamma-glutamyl-glutathione. Natural occurrence and enzymology. J Biol Chem. 1986 Oct 15;261(29):13657–13661. [PubMed] [Google Scholar]
- Adang A. E., Duindam A. J., Brussee J., Mulder G. J., van der Gen A. Synthesis and nucleophilic reactivity of a series of glutathione analogues, modified at the gamma-glutamyl moiety. Biochem J. 1988 Oct 15;255(2):715–720. [PMC free article] [PubMed] [Google Scholar]
- Beale D., Meyer D. J., Taylor J. B., Ketterer B. Evidence that the Yb subunits of hepatic glutathione transferases represent two different but related families of polypeptides. Eur J Biochem. 1983 Dec 1;137(1-2):125–129. doi: 10.1111/j.1432-1033.1983.tb07805.x. [DOI] [PubMed] [Google Scholar]
- Chen W. J., Boehlert C. C., Rider K., Armstrong R. N. Synthesis and characterization of the oxygen and desthio analogues of glutathione as dead-end inhibitors of glutathione S-transferase. Biochem Biophys Res Commun. 1985 Apr 16;128(1):233–240. doi: 10.1016/0006-291x(85)91669-9. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A., Eisenthal R. Statistical considerations in the estimation of enzyme kinetic parameters by the direct linear plot andother methods. Biochem J. 1974 Jun;139(3):721–730. doi: 10.1042/bj1390721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp O., Ladenstein R., Wendel A. The refined structure of the selenoenzyme glutathione peroxidase at 0.2-nm resolution. Eur J Biochem. 1983 Jun 1;133(1):51–69. doi: 10.1111/j.1432-1033.1983.tb07429.x. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Kamisaka K., Habig W. H., Ketley J. N., Arias M., Jakoby W. B. Multiple forms of human glutathione S-transferase and their affinity for bilirubin. Eur J Biochem. 1975 Dec 1;60(1):153–161. doi: 10.1111/j.1432-1033.1975.tb20987.x. [DOI] [PubMed] [Google Scholar]
- Karplus P. A., Schulz G. E. Refined structure of glutathione reductase at 1.54 A resolution. J Mol Biol. 1987 Jun 5;195(3):701–729. doi: 10.1016/0022-2836(87)90191-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Principato G. B., Danielson U. H., Mannervik B. Relaxed thiol substrate specificity of glutathione transferase effected by a non-substrate glutathione derivative. FEBS Lett. 1988 Apr 11;231(1):155–158. doi: 10.1016/0014-5793(88)80722-1. [DOI] [PubMed] [Google Scholar]
- Satoh K., Kitahara A., Sato K. Identification of heterogeneous and microheterogeneous subunits of glutathione S-transferase in rat liver cytosol. Arch Biochem Biophys. 1985 Oct;242(1):104–111. doi: 10.1016/0003-9861(85)90484-9. [DOI] [PubMed] [Google Scholar]
- Sesay M. A., Ammon H. L., Armstrong R. N. Crystallization and a preliminary X-ray diffraction study of isozyme 3-3 of glutathione S-transferase from rat liver. J Mol Biol. 1987 Sep 20;197(2):377–378. doi: 10.1016/0022-2836(87)90133-1. [DOI] [PubMed] [Google Scholar]
- Sugimoto M., Kuhlenkamp J., Ookhtens M., Aw T. Y., Reeve J., Jr, Kaplowitz N. Gamma-glutamylcysteine: a substrate for glutathione S-transferases. Biochem Pharmacol. 1985 Oct 15;34(20):3643–3647. doi: 10.1016/0006-2952(85)90224-2. [DOI] [PubMed] [Google Scholar]
- Tu C. P., Reddy C. C. On the multiplicity of rat liver glutathione S-transferases. J Biol Chem. 1985 Aug 25;260(18):9961–9964. [PubMed] [Google Scholar]
- York M. J., Beilharz G. R., Kuchel P. W. Conformation of reduced glutathione in aqueous solution by 1H and 13C n.m.r. Int J Pept Protein Res. 1987 May;29(5):638–646. doi: 10.1111/j.1399-3011.1987.tb02294.x. [DOI] [PubMed] [Google Scholar]


