Abstract
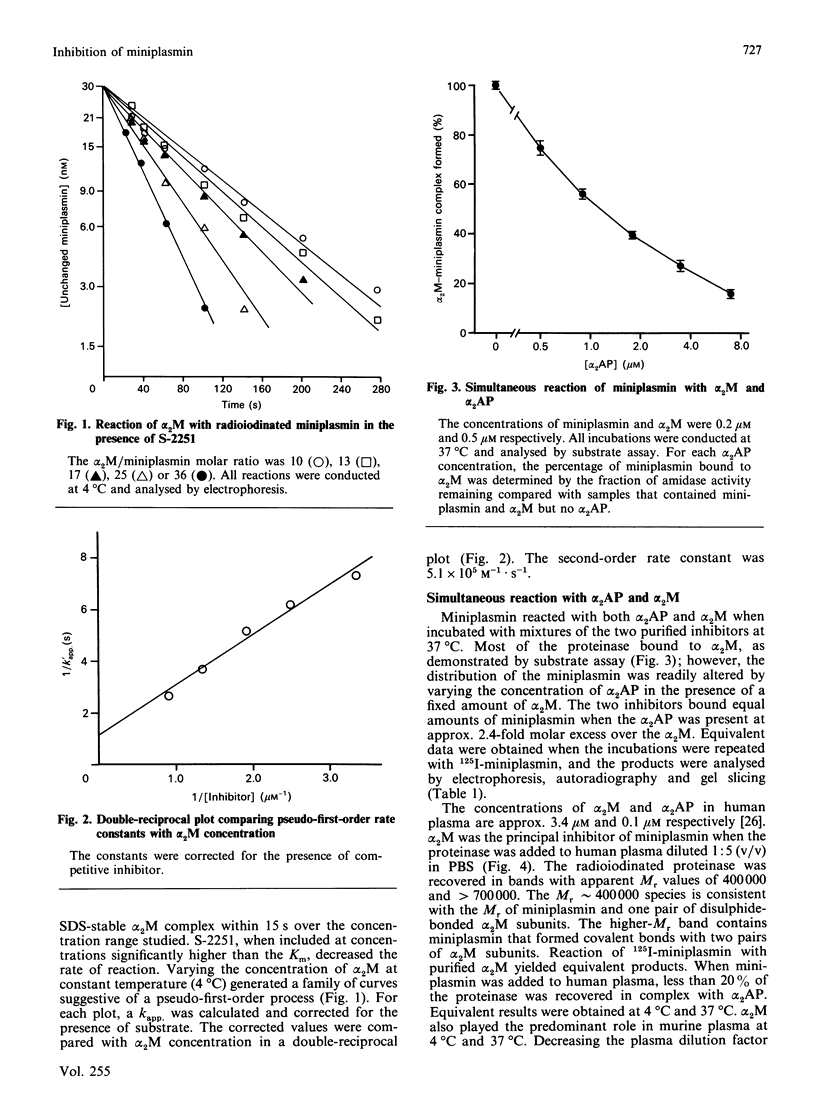
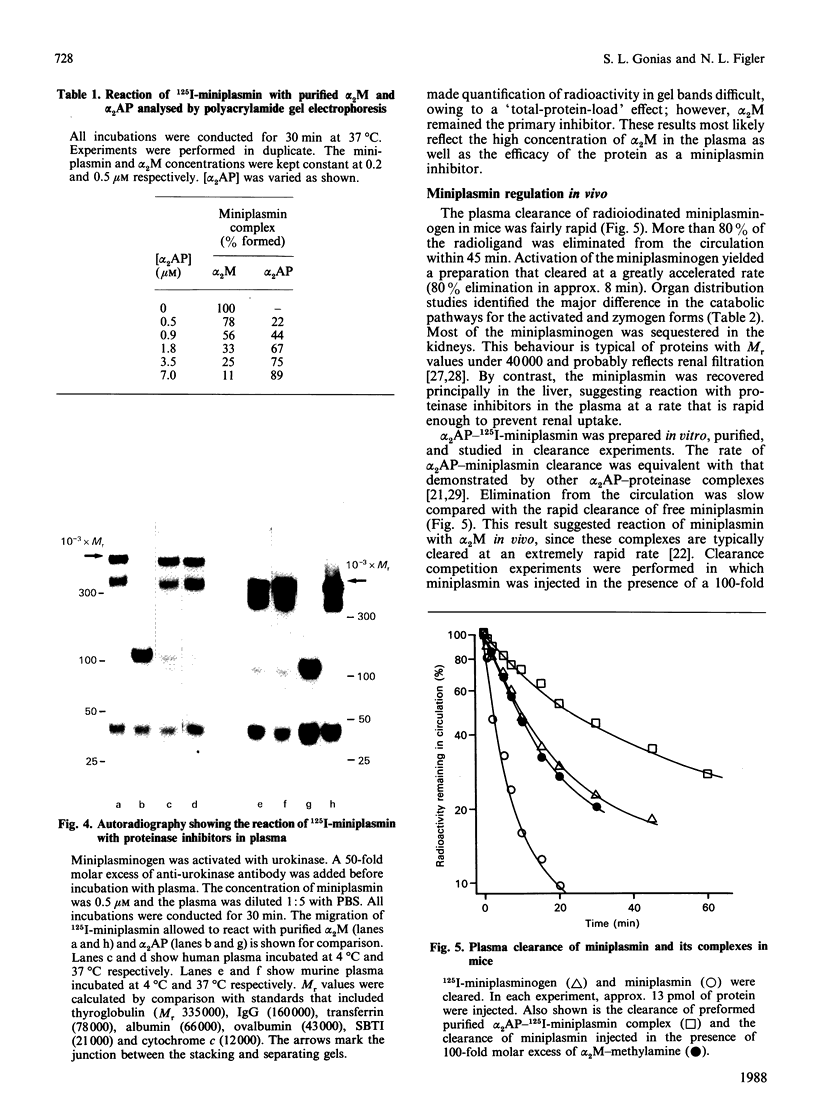
Miniplasmin reacted rapidly with purified human alpha 2-macroglobulin (alpha 2M). More than 98% of the complexes were stabilized by at least one covalent bond. The second-order rate constant for the reaction of alpha 2M with miniplasmin at 4 degrees C was 5.1 x 10(5) M-1.s-1. This value was determined by measuring the formation of covalent alpha 2M-125I-miniplasmin complex; however, the rate constant most likely reflects the bait-region cleavage step in the reaction mechanism. Miniplasmin bound primarily to alpha 2M when incubated at 37 degrees C with various mixtures of alpha 2-antiplasmin (alpha 2AP) and alpha 2M. A 2.4-fold molar excess of alpha 2AP was required to yield an equal distribution of proteinase between the two inhibitors. alpha 2M was the primary miniplasmin inhibitor in human and murine plasma (4 degrees C and 37 degrees C). The extent of covalent-bond formation with murine alpha 2M was approx. 96%. Intravenously injected miniplasmin cleared rapidly from the circulation of mice and was recovered principally in the liver. The catabolic pathway was distinctly different from that of miniplasminogen, which was sequestered mainly in the kidneys. The rate of miniplasmin clearance was much faster than that of purified alpha 2AP-miniplasmin complex, suggesting reaction with alpha 2M in vivo. This was confirmed in clearance competition experiments with alpha 2M-methylamine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki N., Harpel P. C. Inhibitors of the fibrinolytic enzyme system. Semin Thromb Hemost. 1984 Jan;10(1):24–41. doi: 10.1055/s-2007-1004405. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C. O., Gonias S. L., Menapace D. P., Pizzo S. V. A new procedure for the synthesis of polyethylene glycol-protein adducts; effects on function, receptor recognition, and clearance of superoxide dismutase, lactoferrin, and alpha 2-macroglobulin. Anal Biochem. 1983 May;131(1):25–33. doi: 10.1016/0003-2697(83)90131-8. [DOI] [PubMed] [Google Scholar]
- Bieth J. G., Meyer J. F. Temperature and pH dependence of the association rate constant of elastase with alpha 2-macroglobulin. J Biol Chem. 1984 Jul 25;259(14):8904–8906. [PubMed] [Google Scholar]
- Brower M. S., Harpel P. C. Proteolytic cleavage and inactivation of alpha 2-plasmin inhibitor and C1 inactivator by human polymorphonuclear leukocyte elastase. J Biol Chem. 1982 Aug 25;257(16):9849–9854. [PubMed] [Google Scholar]
- Castellino F. J. Biochemistry of human plasminogen. Semin Thromb Hemost. 1984 Jan;10(1):18–23. doi: 10.1055/s-2007-1004404. [DOI] [PubMed] [Google Scholar]
- Christensen U., Sottrup-Jensen L., Magnusson S., Petersen T. E., Clemmensen I. Enzymic properties of the neo-plasmin-Val-422 (miniplasmin). Biochim Biophys Acta. 1979 Apr 12;567(2):472–481. doi: 10.1016/0005-2744(79)90133-5. [DOI] [PubMed] [Google Scholar]
- Christensen U., Sottrup-Jensen L. Mechanism of alpha 2-macroglobulin-proteinase interactions. Studies with trypsin and plasmin. Biochemistry. 1984 Dec 18;23(26):6619–6626. doi: 10.1021/bi00321a052. [DOI] [PubMed] [Google Scholar]
- Clemmensen I., Petersen L. C., Kluft C. Purification and characterization of a novel, oligomeric, plasminogen kringle 4 binding protein from human plasma: tetranectin. Eur J Biochem. 1986 Apr 15;156(2):327–333. doi: 10.1111/j.1432-1033.1986.tb09586.x. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Ganrot P. O. Inhibition of plasmin activity by alpha-2-macroglobulin. Clin Chim Acta. 1967 May;16(2):328–329. doi: 10.1016/0009-8981(67)90201-x. [DOI] [PubMed] [Google Scholar]
- Gonias S. L., Einarsson M., Pizzo S. V. Catabolic pathways for streptokinase, plasmin, and streptokinase activator complex in mice. In vivo reaction of plasminogen activator with alpha 2-macroglobulin. J Clin Invest. 1982 Aug;70(2):412–423. doi: 10.1172/JCI110631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonias S. L., Fuchs H. E., Pizzo S. V. A unique pathway for the plasma elimination of alpha 2-antiplasmin-protease complexes in mice. Thromb Haemost. 1982 Oct 29;48(2):208–210. [PubMed] [Google Scholar]
- Gonias S. L., Pizzo S. V. Conformation and protease binding activity of binary and ternary human alpha 2-macroglobulin-protease complexes. J Biol Chem. 1983 Dec 10;258(23):14682–14685. [PubMed] [Google Scholar]
- Gonias S. L., Pizzo S. V., Hoffman M. Clearance and distribution of recombinant murine gamma-interferon in mice. Cancer Res. 1988 Apr 15;48(8):2021–2024. [PubMed] [Google Scholar]
- Hajjar K. A., Harpel P. C., Jaffe E. A., Nachman R. L. Binding of plasminogen to cultured human endothelial cells. J Biol Chem. 1986 Sep 5;261(25):11656–11662. [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V. Clearance and binding of two electrophoretic "fast" forms of human alpha 2-macroglobulin. J Biol Chem. 1981 Aug 10;256(15):8134–8139. [PubMed] [Google Scholar]
- Knudsen B. S., Silverstein R. L., Leung L. L., Harpel P. C., Nachman R. L. Binding of plasminogen to extracellular matrix. J Biol Chem. 1986 Aug 15;261(23):10765–10771. [PubMed] [Google Scholar]
- Kordich L. C., Porterie V. P., Lago O., Bergonzelli G. E., Sassetti B., Sanchez Avalos J. C. Mini-plasminogen like molecule in septic patients. Thromb Res. 1987 Sep 1;47(5):553–560. doi: 10.1016/0049-3848(87)90360-4. [DOI] [PubMed] [Google Scholar]
- Lijnen H. R., Hoylaerts M., Collen D. Isolation and characterization of a human plasma protein with affinity for the lysine binding sites in plasminogen. Role in the regulation of fibrinolysis and identification as histidine-rich glycoprotein. J Biol Chem. 1980 Nov 10;255(21):10214–10222. [PubMed] [Google Scholar]
- McLellan T. Electrophoresis buffers for polyacrylamide gels at various pH. Anal Biochem. 1982 Oct;126(1):94–99. doi: 10.1016/0003-2697(82)90113-0. [DOI] [PubMed] [Google Scholar]
- Moroz L. A. Mini-plasminogen: a mechanism for leukocyte modulation of plasminogen activation by urokinase. Blood. 1981 Jul;58(1):97–104. [PubMed] [Google Scholar]
- Ney K. A., Pizzo S. V. Fibrinolysis and fibrinogenolysis by Val442-plasmin. Biochim Biophys Acta. 1982 Nov 9;708(2):218–224. doi: 10.1016/0167-4838(82)90223-0. [DOI] [PubMed] [Google Scholar]
- Petersen L. C., Clemmensen I. Kinetics of plasmin inhibition in the presence of a synthetic tripeptide substrate. The reaction with pancreatic trypsin inhibitor and two forms of alpha 2-plasmin inhibitor. Biochem J. 1981 Oct 1;199(1):121–127. doi: 10.1042/bj1990121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo S. V., Rajagopalan S., Roche P. A., Fuchs H. E., Feldman S. R., Gonias S. L. Specificity of alpha 2-macroglobulin covalent cross-linking for the active domain of proteinases. Biol Chem Hoppe Seyler. 1986 Nov;367(11):1177–1182. doi: 10.1515/bchm3.1986.367.2.1177. [DOI] [PubMed] [Google Scholar]
- Powell J. R., Castellino F. J. Activation of human neo-plasminogen-Val442 by urokinase and streptokinase and a kinetic characterization of neoplasmin-Val442. J Biol Chem. 1980 Jun 10;255(11):5329–5335. [PubMed] [Google Scholar]
- Salvesen G. S., Barrett A. J. Covalent binding of proteinases in their reaction with alpha 2-macroglobulin. Biochem J. 1980 Jun 1;187(3):695–701. doi: 10.1042/bj1870695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh B. H., Travis J. The reactive site of human alpha 2-antiplasmin. J Biol Chem. 1987 May 5;262(13):6055–6059. [PubMed] [Google Scholar]
- Steiner J. P., Migliorini M., Strickland D. K. Characterization of the reaction of plasmin with alpha 2-macroglobulin: effect of antifibrinolytic agents. Biochemistry. 1987 Dec 15;26(25):8487–8495. doi: 10.1021/bi00399a068. [DOI] [PubMed] [Google Scholar]
- Wiman B. Affinity-chromatographic purification of human alpha 2-antiplasmin. Biochem J. 1980 Oct 1;191(1):229–232. doi: 10.1042/bj1910229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiman B., Lijnen H. R., Collen D. On the specific interaction between the lysine-binding sites in plasmin and complementary sites in alpha2-antiplasmin and in fibrinogen. Biochim Biophys Acta. 1979 Jul 25;579(1):142–154. doi: 10.1016/0005-2795(79)90094-1. [DOI] [PubMed] [Google Scholar]
- van der Graaf F., Rietveld A., Keus F. J., Bouma B. N. Interaction of human plasma kallikrein and its light chain with alpha 2-macroglobulin. Biochemistry. 1984 Apr 10;23(8):1760–1766. doi: 10.1021/bi00303a027. [DOI] [PubMed] [Google Scholar]