Abstract
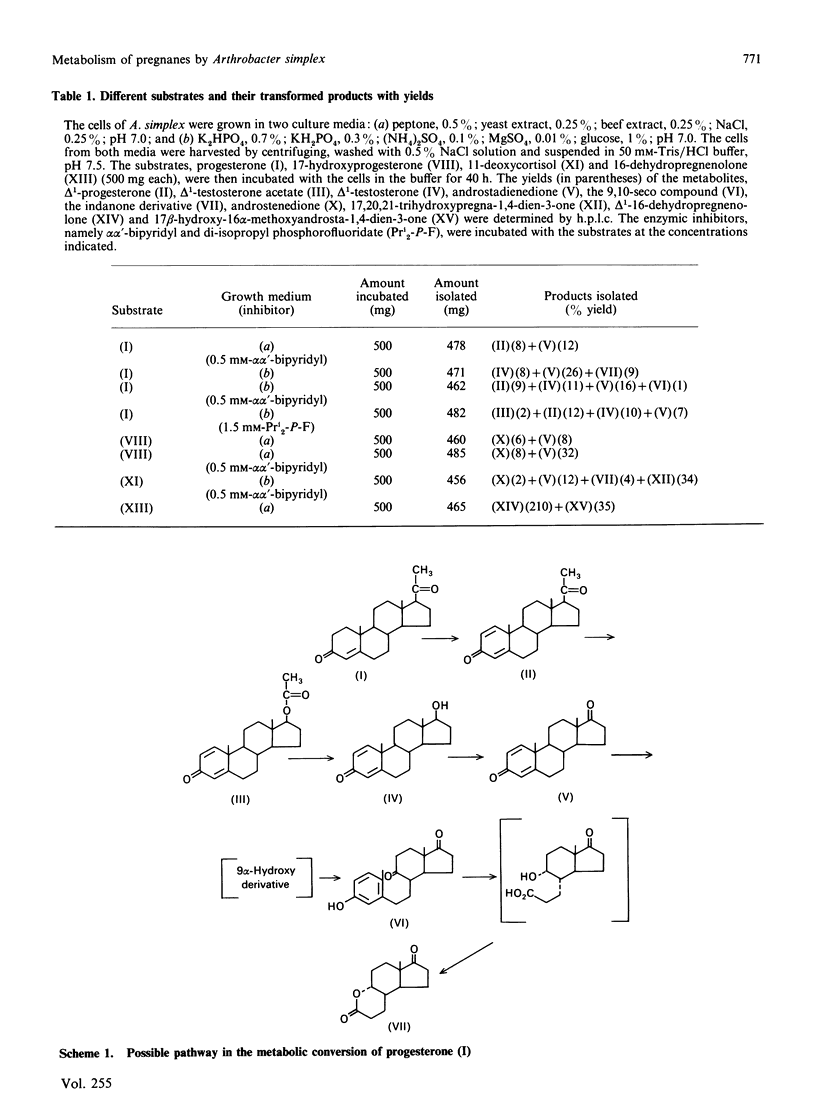
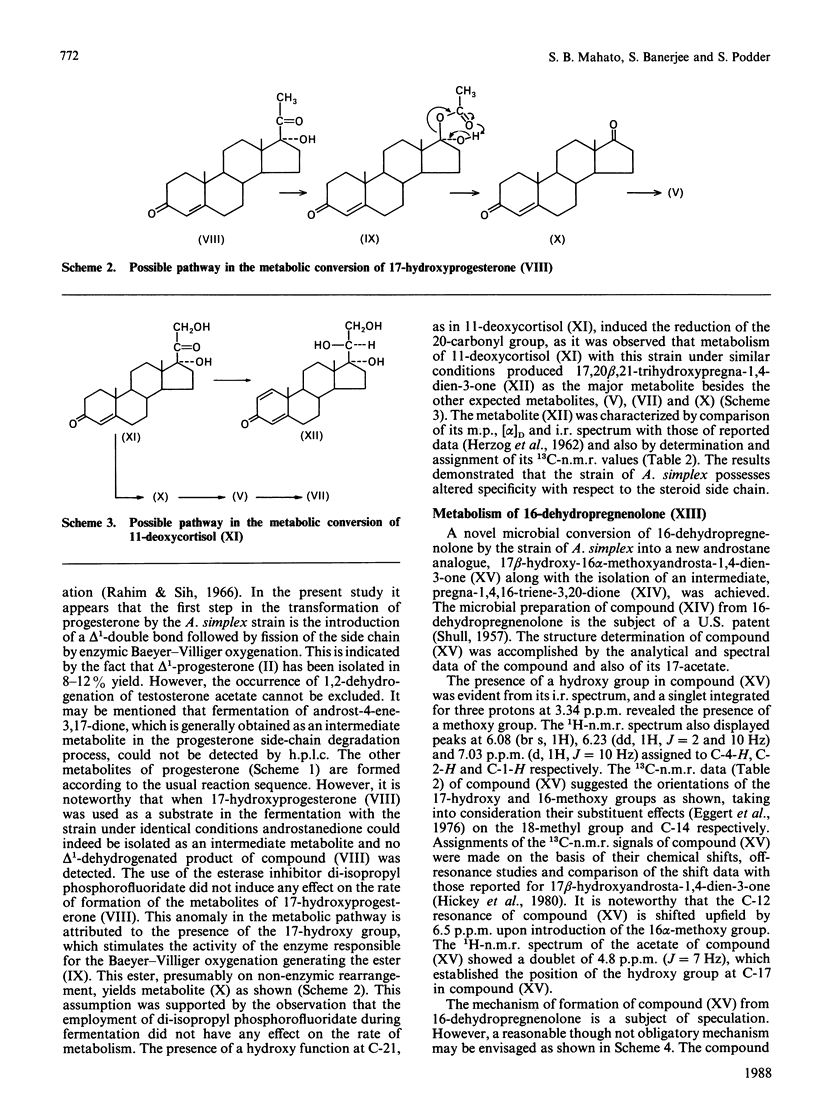
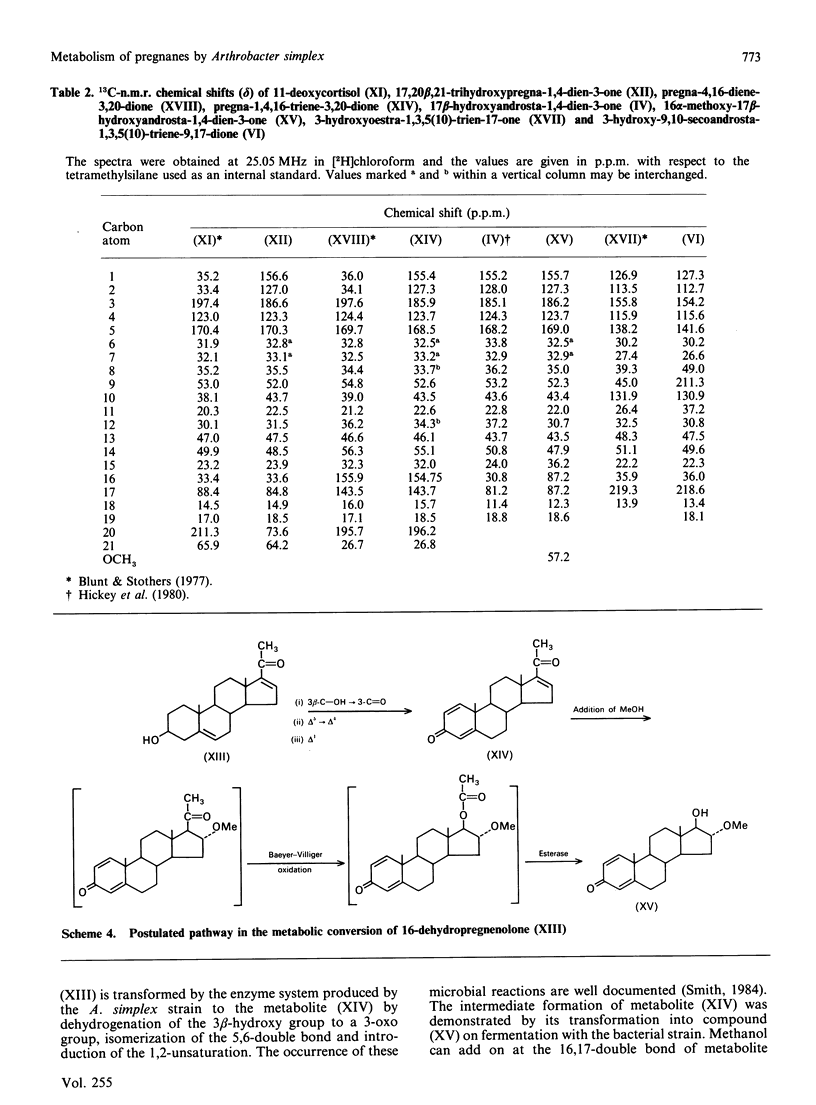
Metabolic processes involving side-chain and ring cleavage of progesterone, 17-hydroxyprogesterone, 11-deoxycortisol and 16-dehydropregnenolone by Arthrobacter simplex were studied. The formation of the metabolites from progesterone indicates a pathway somewhat different from normal in the enzymic reaction sequence, and the 17-hydroxyprogesterone metabolites reveal a non-enzymic rearrangement step. The presence of a hydroxy group at C-21, as in 11-deoxycortisol, induces reduction of the C-20 carbonyl group. The microbial preparation of a novel androstane analogue, 17 beta-hydroxy-16 alpha-methoxyandrosta-1,4-dien-3-one, by incubation of 16-dehydropregnenolone with the bacterial strain was achieved. The formation of this metabolite is a multistep process involving a novel microbial generation of a methoxy group from a double-bond transformation in a steroid skeleton.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Donnelly M. I., Chapman P. J., Dagley S. Bacterial degradation of 3,4,5-trimethoxyphenylacetic and 3-ketoglutaric acids. J Bacteriol. 1981 Aug;147(2):477–481. doi: 10.1128/jb.147.2.477-481.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert H., VanAntwerp C. L., Bhacca N. S., Djerassi C. Carbon-13 nuclear magnetic resonance spectra of hydroxy steroids. J Org Chem. 1976 Jan 9;41(1):71–78. doi: 10.1021/jo00863a016. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Wang K. C., Sih C. J., Whitlock H., Jr Mechanisms of steroid oxidation by microorganisms. IX. On the mechanism of ring A cleavage in the degradation of 9,10-seco steroids by microorganisms. J Biol Chem. 1966 Feb 10;241(3):551–559. [PubMed] [Google Scholar]
- Lee S. S., Sih C. J. Mechanisms of steroid oxidation by microorganisms. XII. Metabolism of hexahydroindanpropionic acid derivatives. Biochemistry. 1967 May;6(5):1395–1403. doi: 10.1021/bi00857a023. [DOI] [PubMed] [Google Scholar]
- Martin C. K. Microbial cleavage of sterol side chains. Adv Appl Microbiol. 1977;22:29–58. doi: 10.1016/s0065-2164(08)70159-x. [DOI] [PubMed] [Google Scholar]
- Rahm M. A., Sih C. J. Mechanisms of steroid oxidation by microorganisms. XI. Enzymatic cleavage of the pregnane side chain. J Biol Chem. 1966 Aug 10;241(15):3615–3623. [PubMed] [Google Scholar]
- Sih C. J., Lee S. S., Tsong Y. Y., Wang K. C. Mechanisms of steroid oxidation by microorganisms. 8. 3,4-Dihydroxy-9,10-secoandrosta-1,3,5(10)-triene-9,17-dione, an intermediate in the microbiological degradation of ring A of androst-4-ene-3,17-dione. J Biol Chem. 1966 Feb 10;241(3):540–550. [PubMed] [Google Scholar]
- WANG K. C., SIH C. J. MECHANISMS OF STEROID OXIDATION BY MICROORGANISMS. IV. SECO INTERMEDIATES. Biochemistry. 1963 Nov-Dec;2:1238–1243. doi: 10.1021/bi00906a011. [DOI] [PubMed] [Google Scholar]


