Abstract
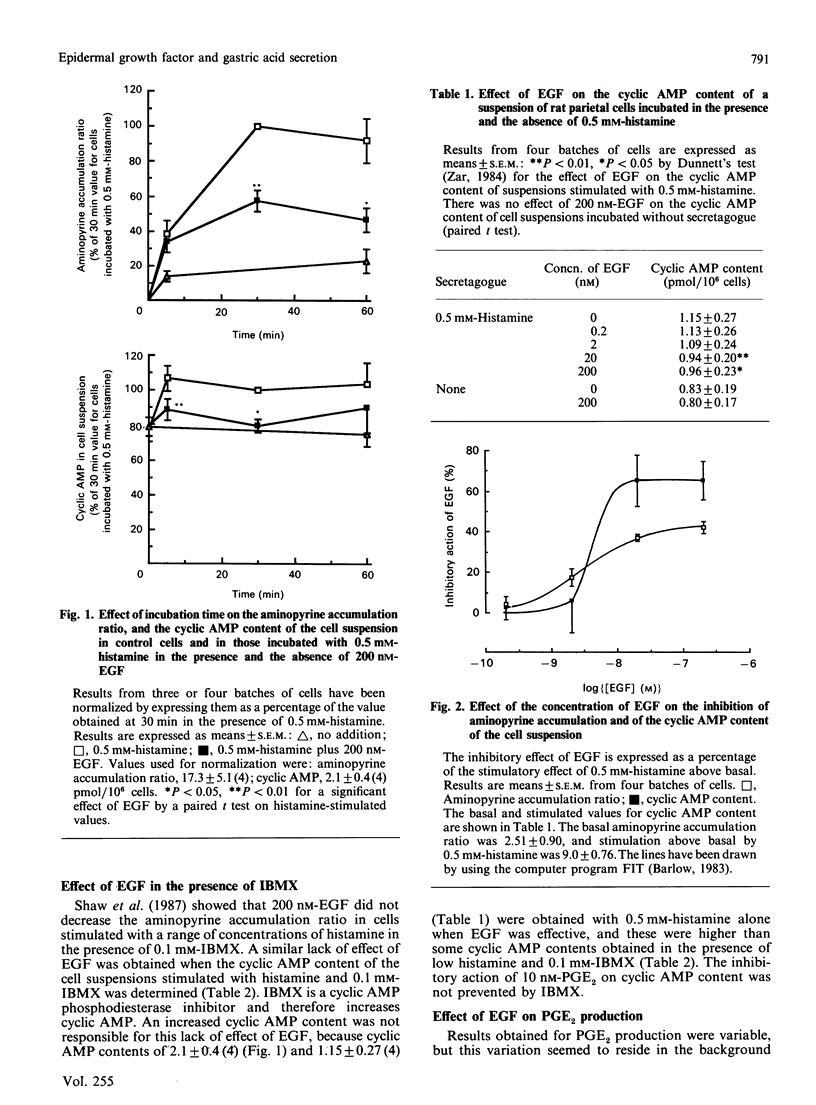
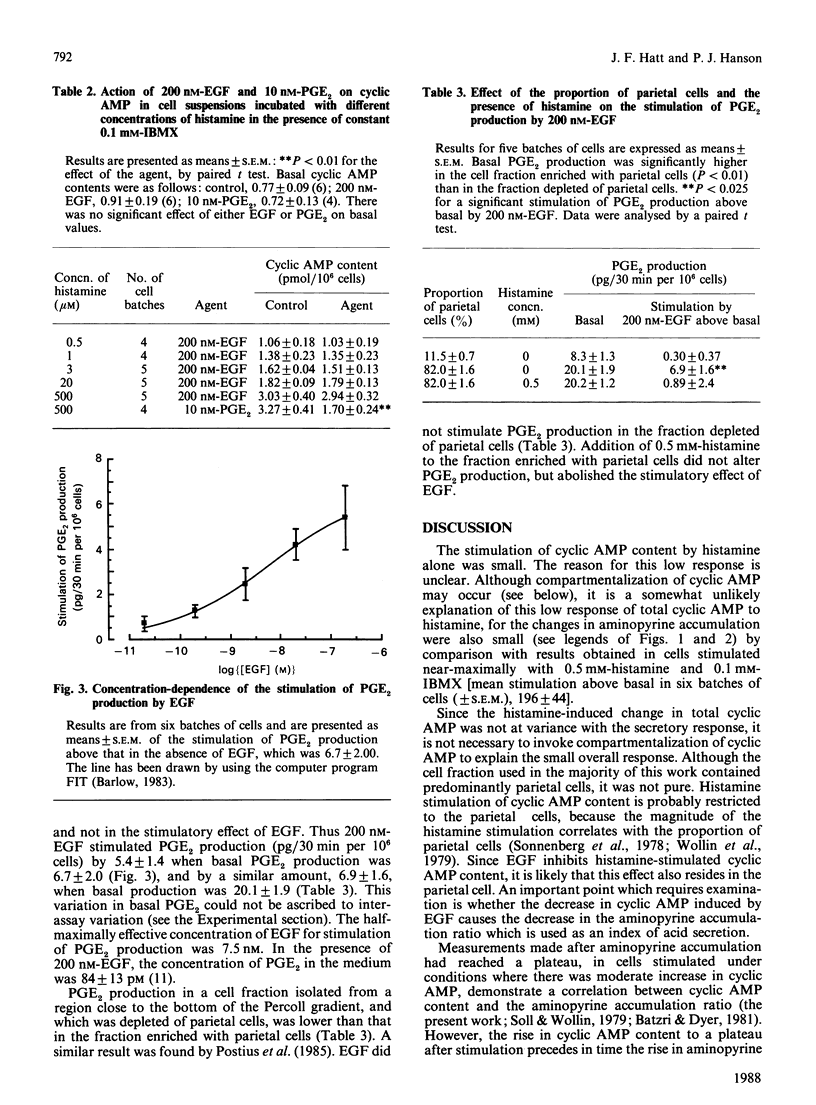
Histamine (0.5 mM) stimulated the cyclic AMP content of cell suspensions containing greater than 80% parietal cells. Epidermal growth factor (EGF) inhibited this stimulatory effect of histamine, but had no effect on basal cyclic AMP content. The half-maximally effective concentration of EGF for inhibition of histamine-stimulated cyclic AMP was 3.9 nM. The equivalent measurement for the inhibition of histamine-stimulated aminopyrine accumulation was 3.0 nM. Aminopyrine accumulation was measured because it provides an index of the secretory activity of the cell. The cyclic AMP phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) prevented the inhibitory effect of EGF on cyclic AMP content. This effect of IBMX was not caused by its ability to raise cellular cyclic AMP content in the presence of histamine. Prevention by IBMX of the inhibitory action of EGF on histamine-stimulated aminopyrine accumulation had been shown previously [Shaw, Hatt, Anderson & Hanson (1987) Biochem. J. 244, 699-704]. EGF stimulated prostaglandin E2 (PGE2) production in the cell fraction containing greater than 80% parietal cells, with the half-maximally effective concentration being 7.5 nM. EGF was ineffective in stimulating PGE2 production if the cell fraction was depleted of parietal cells (12%), or if 0.5 mM-histamine was added to the enriched parietal-cell fraction. In conclusion, EGF may inhibit histamine-stimulated acid secretion by decreasing the cyclic AMP content of parietal cells. This effect could be mediated by an increase in cyclic AMP phosphodiesterase activity, but it is unlikely to involve an effect of EGF on parietal-cell prostaglandin production.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Hanson P. J. Involvement of calcium-sensitive phospholipid-dependent protein kinase in control of acid secretion by isolated rat parietal cells. Biochem J. 1985 Dec 1;232(2):609–611. doi: 10.1042/bj2320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batzri S., Dyer J. Aminopyrine uptake by guinea pig gastric mucosal cells. Mediation by cyclic AMP and interactions among secretagogues. Biochim Biophys Acta. 1981 Jul 17;675(3-4):416–426. doi: 10.1016/0304-4165(81)90035-0. [DOI] [PubMed] [Google Scholar]
- Blum H., Chance B., Litchfield W. J. Effect of pH on bovine liver catalase as determined by electron paramagnetic resonance. Biochim Biophys Acta. 1978 Jun 21;534(2):317–321. doi: 10.1016/0005-2795(78)90014-4. [DOI] [PubMed] [Google Scholar]
- Bower J. M., Camble R., Gregory H., Gerring E. L., Willshire I. R. The inhibition of gastric acid secretion by epidermal growth factor. Experientia. 1975 Jul 15;31(7):825–826. doi: 10.1007/BF01938488. [DOI] [PubMed] [Google Scholar]
- Chew C. S. Parietal cell protein kinases. Selective activation of type I cAMP-dependent protein kinase by histamine. J Biol Chem. 1985 Jun 25;260(12):7540–7550. [PubMed] [Google Scholar]
- Chiba T., Hirata Y., Taminato T., Kadowaki S., Matsukura S., Fujita T. Epidermal growth factor stimulates prostaglandin E release from isolated perfused rat stomach. Biochem Biophys Res Commun. 1982 Mar 15;105(1):370–374. doi: 10.1016/s0006-291x(82)80054-5. [DOI] [PubMed] [Google Scholar]
- Dembiński A., Drozdowicz D., Gregory H., Konturek S. J., Warzecha Z. Inhibition of acid formation by epidermal growth factor in the isolated rabbit gastric glands. J Physiol. 1986 Sep;378:347–357. doi: 10.1113/jphysiol.1986.sp016223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembiński A., Gregory H., Konturek S. J., Polański M. Trophic action of epidermal growth factor on the pancreas and gastroduodenal mucosa in rats. J Physiol. 1982 Apr;325:35–42. doi: 10.1113/jphysiol.1982.sp014133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory H. In vivo aspects of urogastrone-epidermal growth factor. J Cell Sci Suppl. 1985;3:11–17. doi: 10.1242/jcs.1985.supplement_3.2. [DOI] [PubMed] [Google Scholar]
- Hassid A. Regulation of prostaglandin biosynthesis in cultured cells. Am J Physiol. 1982 Nov;243(5):C205–C211. doi: 10.1152/ajpcell.1982.243.5.C205. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Brzozowski T., Piastucki I., Dembinski A., Radecki T., Dembinska-Kiec A., Zmuda A., Gregory H. Role of mucosal prostaglandins and DNA synthesis in gastric cytoprotection by luminal epidermal growth factor. Gut. 1981 Nov;22(11):927–932. doi: 10.1136/gut.22.11.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postius S., Ruoff H. J., Szelenyi I. Prostaglandin formation by isolated gastric parietal and nonparietal cells of the rat. Br J Pharmacol. 1985 Apr;84(4):871–877. doi: 10.1111/j.1476-5381.1985.tb17381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puurunen J., Lohse M. J., Schwabe U. Interactions between intracellular cyclic AMP and agonist-induced inositol phospholipid breakdown in isolated gastric mucosal cells of the rat. Naunyn Schmiedebergs Arch Pharmacol. 1987 Nov;336(5):471–477. doi: 10.1007/BF00169301. [DOI] [PubMed] [Google Scholar]
- Schepp W., Steffen B., Schusdziarra V., Classen M. Calcium, calmodulin, and cyclic adenosine monophosphate modulate prostaglandin E2 release from isolated human gastric mucosal cells. J Clin Endocrinol Metab. 1986 Oct;63(4):886–891. doi: 10.1210/jcem-63-4-886. [DOI] [PubMed] [Google Scholar]
- Shaw G. P., Anderson N. G., Hanson P. J. Metabolism and gastric acid secretion. Substrate-dependency of aminopyrine accumulation in isolated rat parietal cells. Biochem J. 1985 Apr 1;227(1):223–229. doi: 10.1042/bj2270223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G. P., Hatt J. F., Anderson N. G., Hanson P. J. Action of epidermal growth factor on acid secretion by rat isolated parietal cells. Biochem J. 1987 Jun 15;244(3):699–704. doi: 10.1042/bj2440699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner K. A., Soper B. D., Tepperman B. L. Effect of sialoadenectomy and salivary gland extracts on gastrointestinal mucosal growth and gastrin levels in the rat. J Physiol. 1984 Jun;351:1–12. doi: 10.1113/jphysiol.1984.sp015227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll A. H., Chen M. C., Amirian D. A., Toomey M., Alvarez R. Prostanoid inhibition of canine parietal cells. Am J Med. 1986 Aug 18;81(2A):5–11. doi: 10.1016/s0002-9343(86)80003-1. [DOI] [PubMed] [Google Scholar]
- Soll A. H. Secretagogue stimulation of [14C]aminopyrine accumulation by isolated canine parietal cells. Am J Physiol. 1980 Apr;238(4):G366–G375. doi: 10.1152/ajpgi.1980.238.4.G366. [DOI] [PubMed] [Google Scholar]
- Soll A. H., Whittle B. J. Prostacyclin analogues inhibit canine parietal cell activity and cyclic AMP formation. Prostaglandins. 1981 Feb;21(2):353–365. doi: 10.1016/0090-6980(81)90153-2. [DOI] [PubMed] [Google Scholar]
- Soll A. H., Wollin A. Histamine and cyclic AMP in isolated canine parietal cells. Am J Physiol. 1979 Nov;237(5):E444–E450. doi: 10.1152/ajpendo.1979.237.5.E444. [DOI] [PubMed] [Google Scholar]
- Wollin A., Soll A. H., Samloff I. M. Actions of histamine, secretin, and PGE2 on cyclic AMP production by isolated canine fundic mucosal cells. Am J Physiol. 1979 Nov;237(5):E437–E443. doi: 10.1152/ajpendo.1979.237.5.E437. [DOI] [PubMed] [Google Scholar]


