Abstract
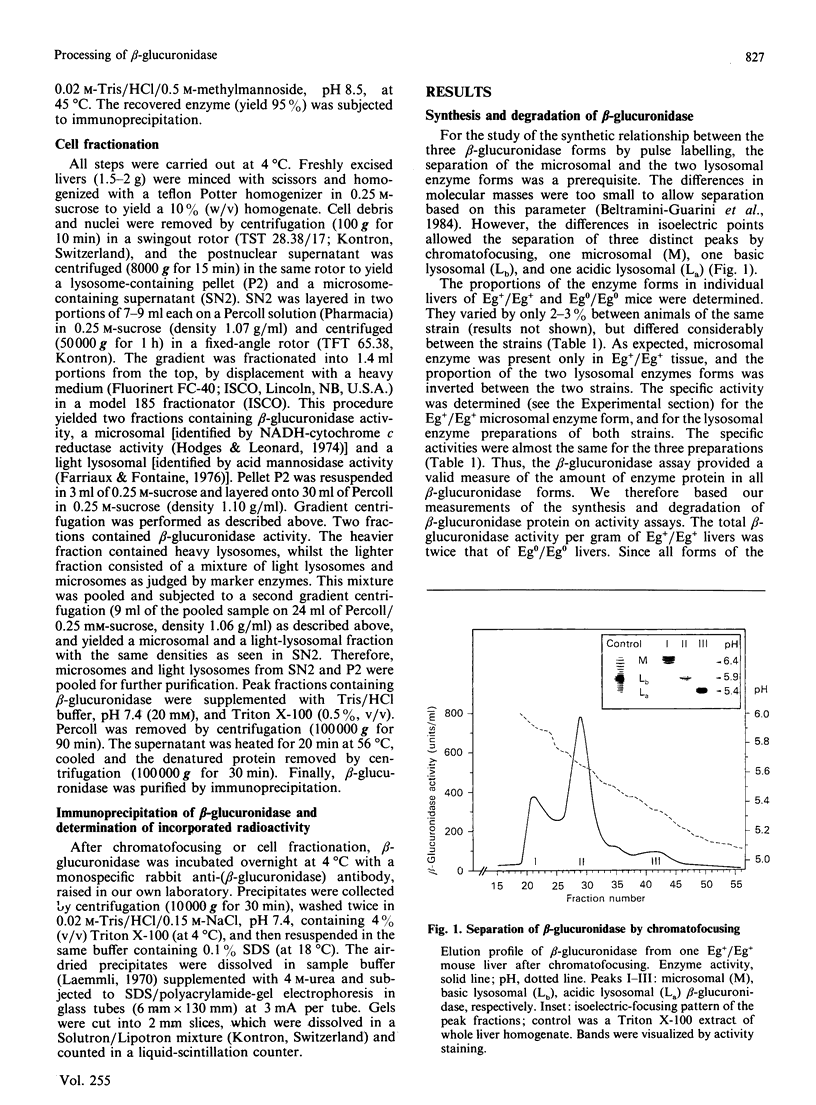
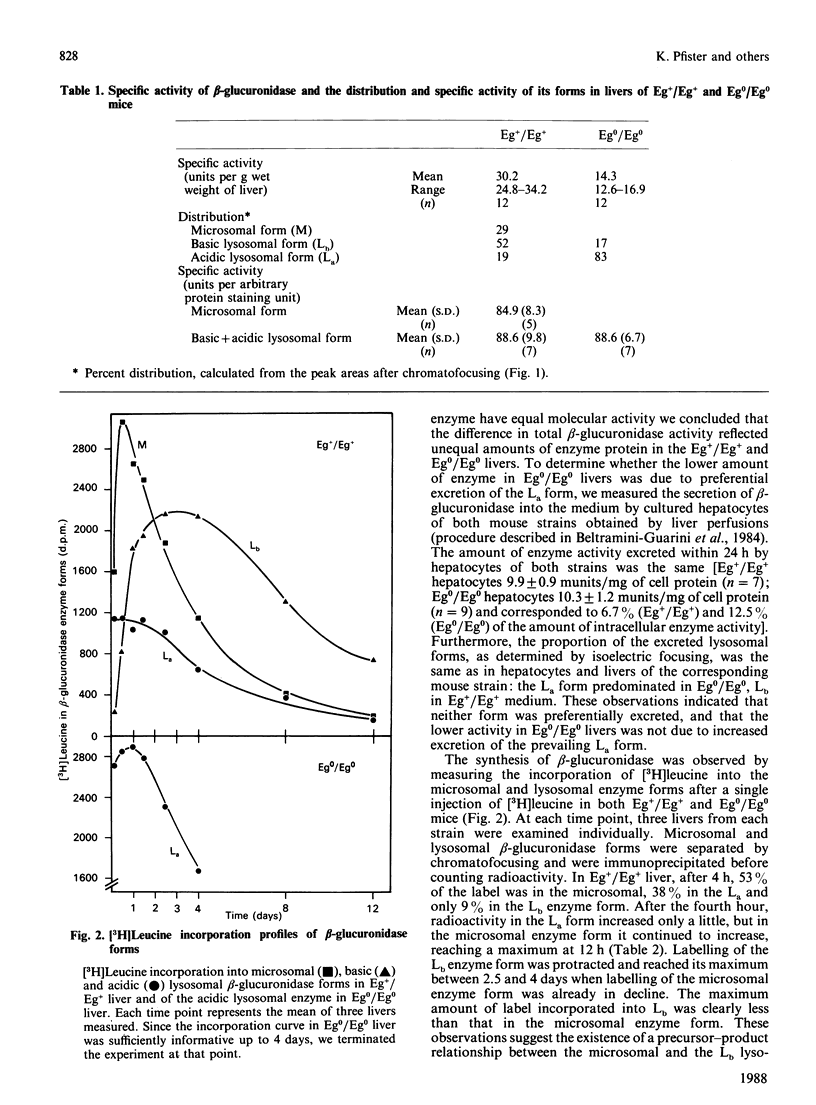
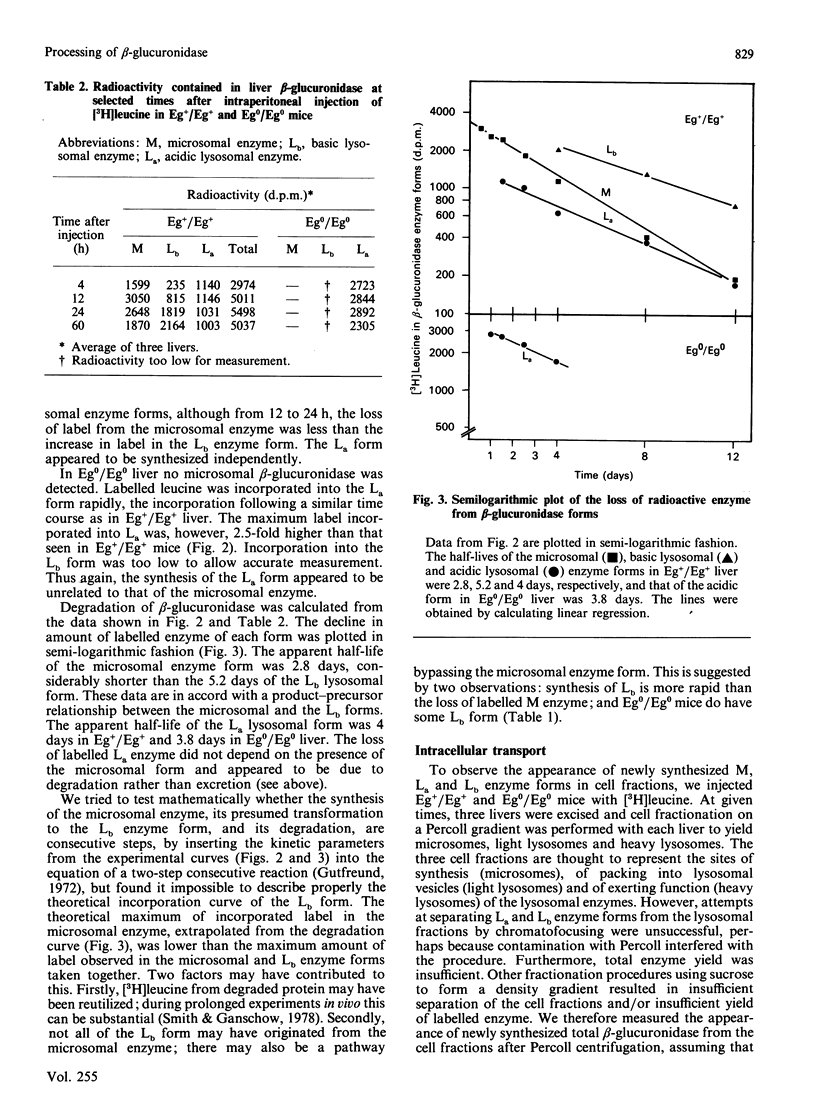
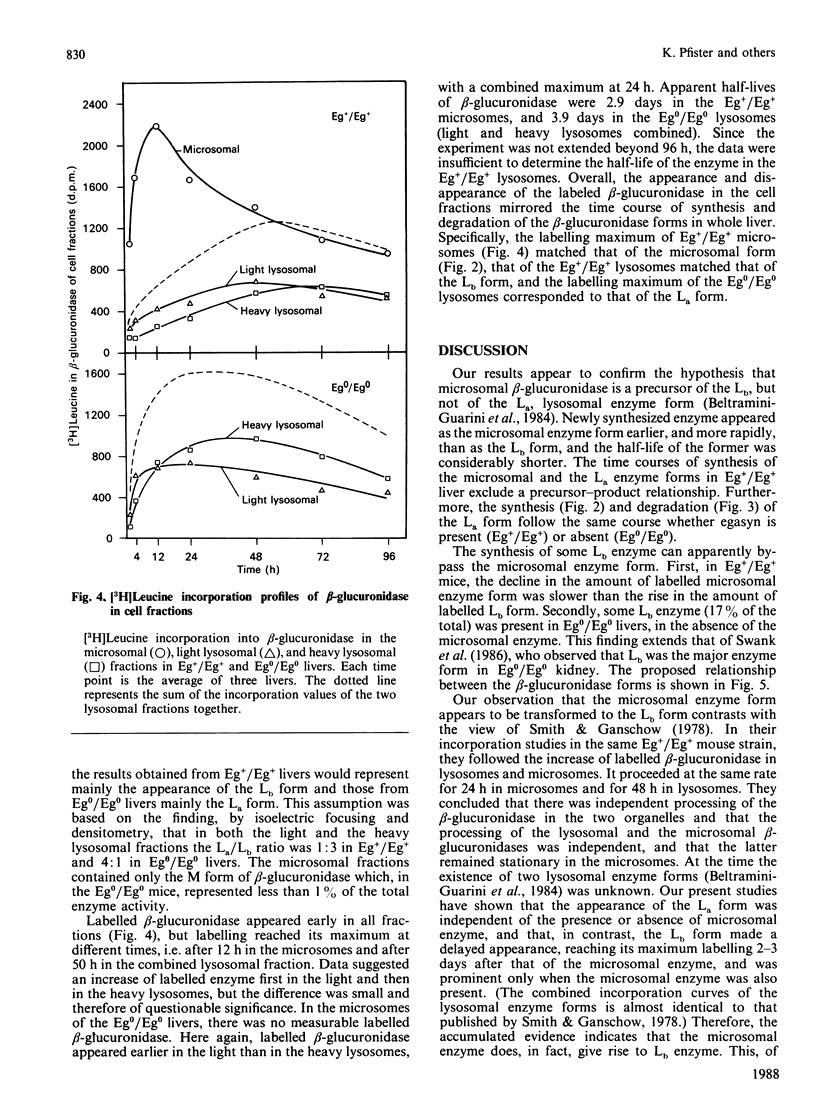
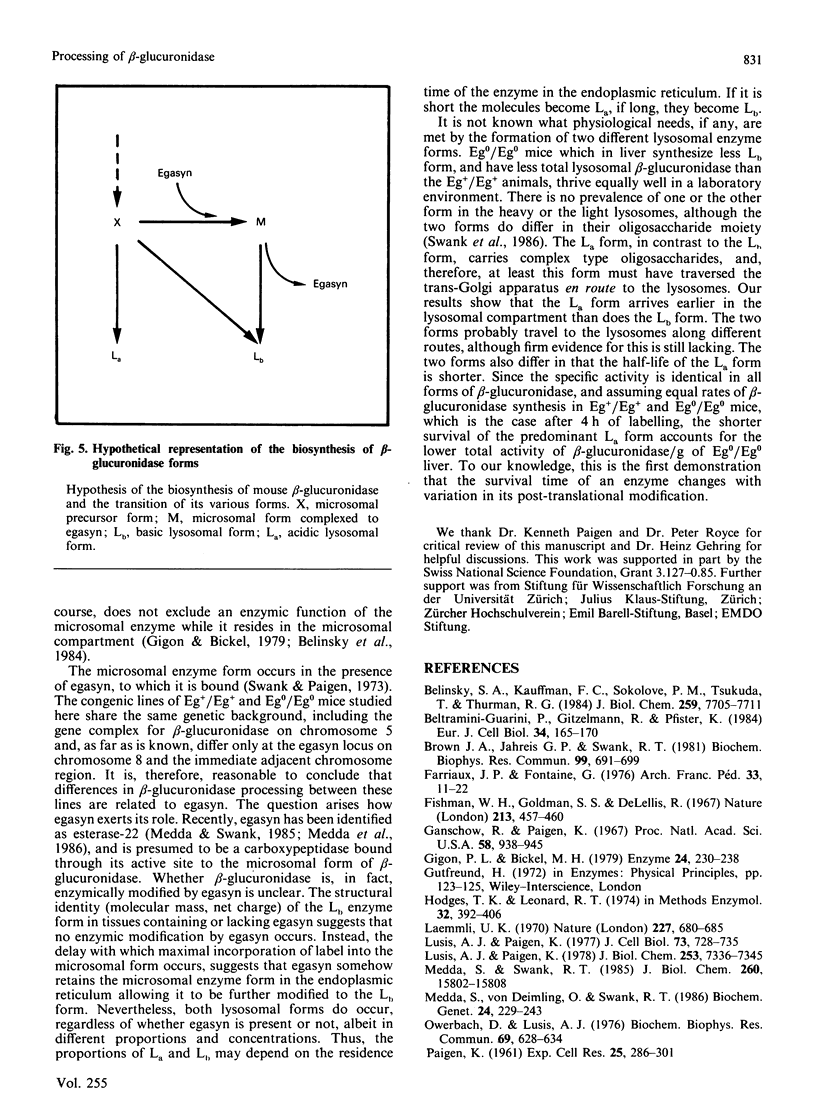
Three differently modified forms of beta-glucuronidase are known to exist: a microsomal enzyme form (M) existing in tissues where egasyn, a second microsomal protein, is present; and an acidic (La; complex-type oligosaccharide) and a basic (Lb; non-complex type oligosaccharide) lysosomal form which occur in all mouse tissues. Lb predominates in tissues containing microsomal beta-glucuronidase, La in those lacking it. In pulse-labelling experiments using mouse strain C57BL/6 liver containing egasyn (Eg+/Eg+) and microsomal enzyme, about half of the newly synthesized beta-glucuronidase was processed to the microsomal enzyme form, which was evidently further processed to Lb, and about half directly to La. In contrast, in liver of the congenic line C57BL/6.YBR Es-1b Eg0 that lacks egasyn (Eg0/Eg0) and microsomal enzyme, most of the labelled beta-glucuronidase was processed to La, and only a minor portion to Lb. Newly synthesized enzyme appeared first in microsomal, then in light and heavy lysosomal fractions of Eg+/Eg+ liver. In Eg0/Eg0 liver, no labelled enzyme was measurable in the microsomes, but it appeared rapidly in both types of lysosomes. Taken together these findings indicate that the microsomal enzyme form serves as a precursor of Lb, and that La is synthesized independently. The apparent half-life of La is only two-thirds that of Lb; this fact accounts for the reduced beta-glucuronidase activity in Eg0/Eg0 liver, which contains La as the predominant form.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belinsky S. A., Kauffman F. C., Sokolove P. M., Tsukuda T., Thurman R. G. Calcium-mediated inhibition of glucuronide production by epinephrine in the perfused rat liver. J Biol Chem. 1984 Jun 25;259(12):7705–7711. [PubMed] [Google Scholar]
- Beltramini-Guarini P., Gitzelmann R., Pfister K. Presence and absence of the microsomal beta-glucuronidase in mice correlates with differences in the processing of the lysosomal enzyme. Eur J Cell Biol. 1984 May;34(1):165–170. [PubMed] [Google Scholar]
- Brown J. A., Jahreis G. P., Swank R. T. The synthesis and processing of beta-glucuronidase in normal and egasyn deficient mouse kidney. Biochem Biophys Res Commun. 1981 Mar 31;99(2):691–699. doi: 10.1016/0006-291x(81)91799-x. [DOI] [PubMed] [Google Scholar]
- Farriaux J. P., Fontaine G. La mannosidose: un diagnostic simple. Arch Fr Pediatr. 1976 Jan;33(1):11–22. [PubMed] [Google Scholar]
- Fishman W. H., Goldman S. S., DeLellis R. Dual localization of beta-glucuronidase in endoplasmic reticulum and in lysosomes. Nature. 1967 Feb 4;213(5075):457–460. doi: 10.1038/213457a0. [DOI] [PubMed] [Google Scholar]
- Ganschow R., Paigen K. Separate genes determining the structure and intracellular location of hepatic glucuronidase. Proc Natl Acad Sci U S A. 1967 Sep;58(3):938–945. doi: 10.1073/pnas.58.3.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigon P. L., Bickel M. H. Interference of UDP-glucuronyltransferase and beta-glucuronidase activity in rat liver microsomes at pH 7.5 with p-nitrophenol and p-nitrophenylglucuronide as substrates. Enzyme. 1979;24(4):230–238. doi: 10.1159/000458664. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lusis A. J., Paigen K. Relationships between levels of membrane-bound glucuronidase and the associated protein egasyn in mouse tissues. J Cell Biol. 1977 Jun;73(3):728–735. doi: 10.1083/jcb.73.3.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusis A. J., Paigen K. The large scale isolation of mouse beta-glucuronidase and comparison of allozymes. J Biol Chem. 1978 Oct 25;253(20):7336–7345. [PubMed] [Google Scholar]
- Medda S., Swank R. T. Egasyn, a protein which determines the subcellular distribution of beta-glucuronidase, has esterase activity. J Biol Chem. 1985 Dec 15;260(29):15802–15808. [PubMed] [Google Scholar]
- Medda S., von Deimling O., Swank R. T. Identity of esterase-22 and egasyn, the protein which complexes with microsomal beta-glucuronidase. Biochem Genet. 1986 Apr;24(3-4):229–243. doi: 10.1007/BF00502791. [DOI] [PubMed] [Google Scholar]
- Owerbach D., Luis A. J. Phenobarbital induction of egasyn: availability of egasyn in vivo determines glucuronidase binding to membrane. Biochem Biophys Res Commun. 1976 Apr 5;69(3):628–634. doi: 10.1016/0006-291x(76)90922-0. [DOI] [PubMed] [Google Scholar]
- PAIGEN K. The effect of mutation on the intracellular location of beta-glucuronidase. Exp Cell Res. 1961 Nov;25:286–301. doi: 10.1016/0014-4827(61)90280-4. [DOI] [PubMed] [Google Scholar]
- Paigen K. Acid hydrolases as models of genetic control. Annu Rev Genet. 1979;13:417–466. doi: 10.1146/annurev.ge.13.120179.002221. [DOI] [PubMed] [Google Scholar]
- Smith K., Ganschow R. E. Turnover of murine beta-glucuronidase. Comparison among liver, kidney, and spleen and between lysosomes and microsomes. J Biol Chem. 1978 Aug 10;253(15):5437–5442. [PubMed] [Google Scholar]
- Strawser L. D., Touster O. The cellular processing of lysosomal enzymes and related proteins. Rev Physiol Biochem Pharmacol. 1980;87:169–210. doi: 10.1007/BFb0030898. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Paigen K. Biochemical and genetic evidence for a macromolecular -glucuronidase complex in microsomal membranes. J Mol Biol. 1973 Jul 5;77(3):371–389. doi: 10.1016/0022-2836(73)90445-2. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Pfister K., Miller D., Chapman V. The egasyn gene affects the processing of oligosaccharides of lysosomal beta-glucuronidase in liver. Biochem J. 1986 Dec 1;240(2):445–454. doi: 10.1042/bj2400445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominco S., Paigen K. Egasyn, a protein complexed with microsomal beta-glucuronidase. J Biol Chem. 1975 Feb 10;250(3):1146–1148. [PubMed] [Google Scholar]
- Tsuji H., Kato K. The synthesis of rat liver lysosomes. II. Intracellular transport of beta-glucuronidase. J Biochem. 1977 Sep;82(3):637–644. doi: 10.1093/oxfordjournals.jbchem.a131738. [DOI] [PubMed] [Google Scholar]