Abstract
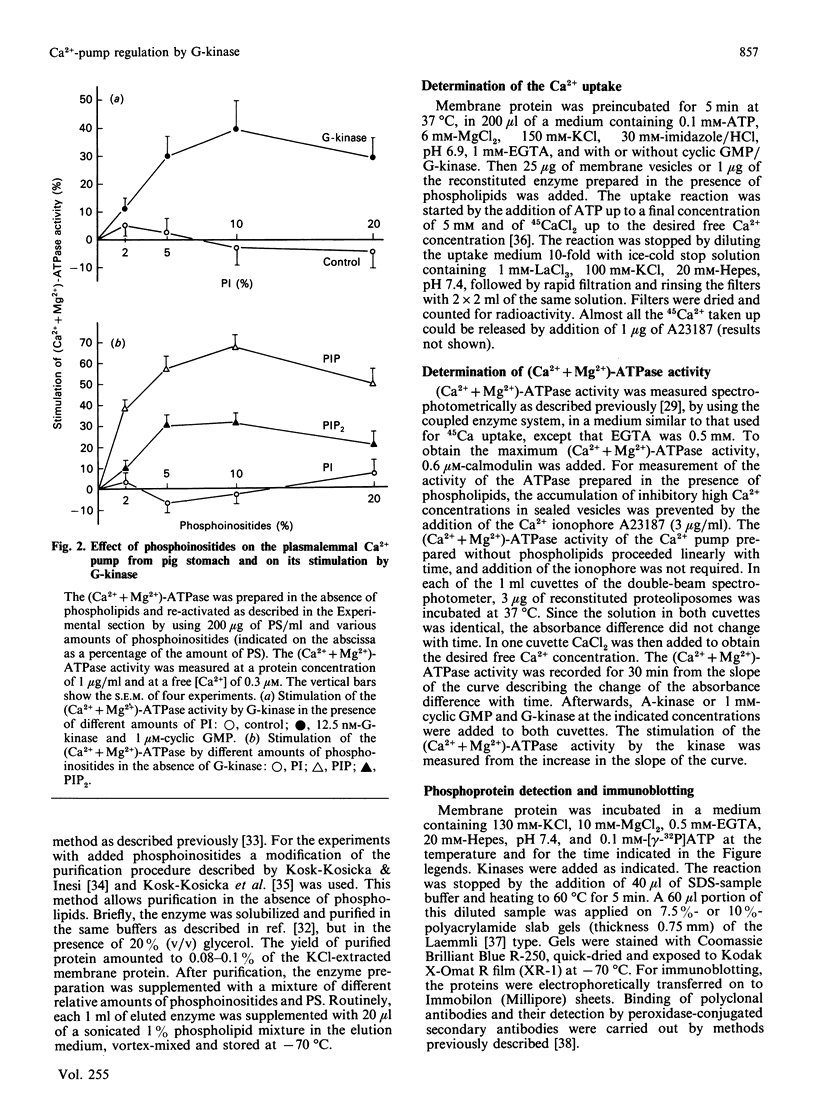
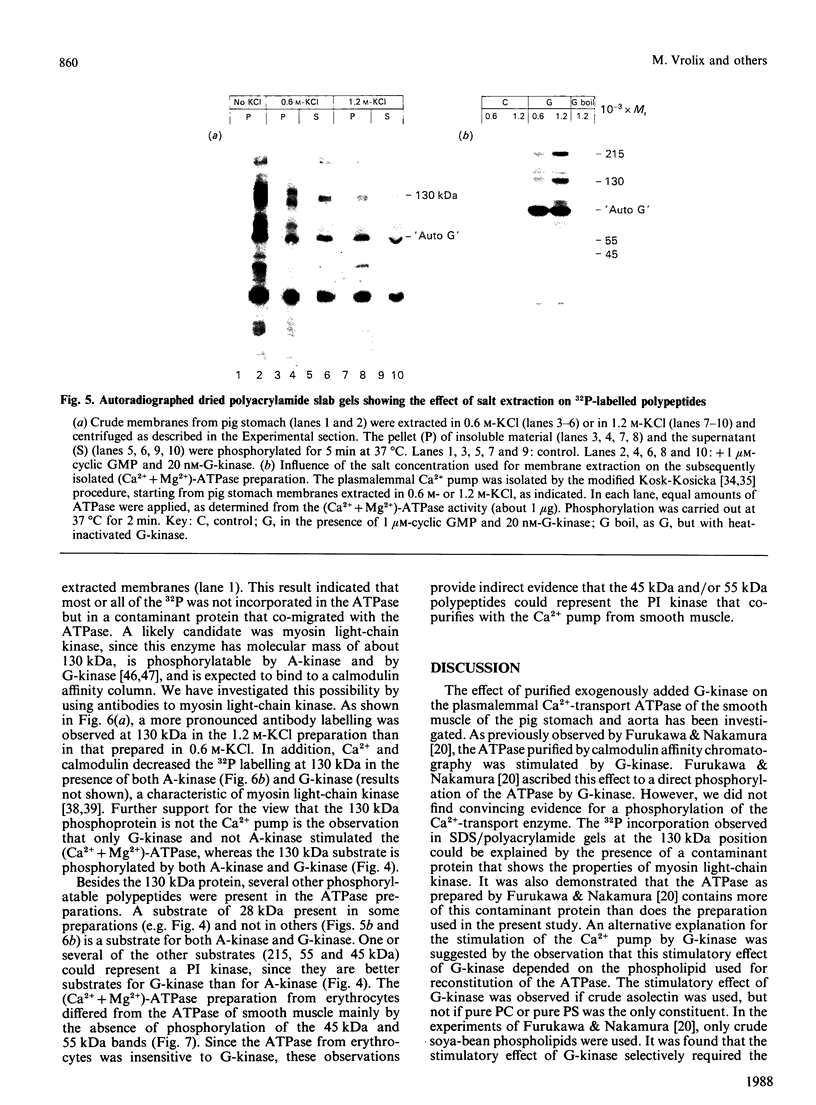
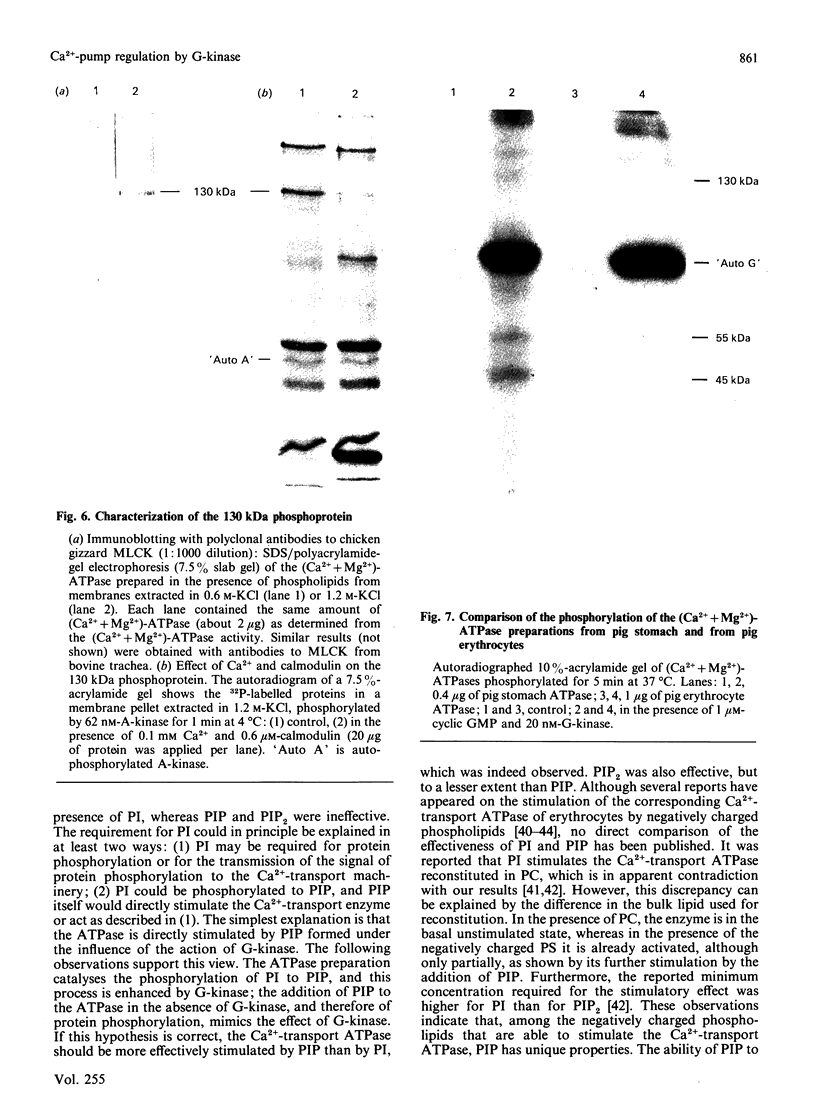
The effect of phosphorylation by cyclic GMP-dependent protein kinase (G-kinase) on the activity of the plasmalemmal Ca2+-transport ATPase was studied on isolated plasma membranes and on the ATPase purified from pig erythrocytes and from the smooth muscle of pig stomach and pig aorta. Incubation with G-kinase resulted, in both smooth-muscle preparations, but not in the erythrocyte ATPase, in a higher Ca2+ affinity and in an increase in the maximal rate of Ca2+ uptake. Cyclic AMP-dependent protein kinase (A-kinase) did not exert such an effect. The stimulation of the (Ca2+ + Mg2+)-dependent ATPase activity of the purified Ca2+ pump reconstituted in liposomes depended on the phospholipid used for reconstitution. The stimulation of the (Ca2+ + Mg2+)-ATPase activity by G-kinase was only observed in the presence of phosphatidylinositol (PI). G-kinase, but not A-kinase, stimulated the phosphorylation of PI to phosphatidylinositol phosphate (PIP) in a preparation of (Ca2+ + Mg2+)-ATPase obtained by calmodulin affinity chromatography from smooth muscle, but not in a similar preparation from erythrocytes. Adenosine inhibited both the phosphorylation of PI and the stimulation of the (Ca2+ + Mg2+)-ATPase by G-kinase. In the absence of G-kinase the (Ca2+ + Mg2+)-ATPase was stimulated by the addition of PIP, but not by PI. In contrast with previous results of Furukawa & Nakamura [(1987) J. Biochem (Tokyo) 101, 287-290], no convincing evidence for a phosphorylation of the (Ca2+ + Mg2+)-ATPase was found. Evidence is presented showing that the apparent phosphorylation occurs in a contaminant protein, possibly myosin light-chain kinase. It is proposed that G-kinase stimulates the plasmalemmal Ca2+ pump of smooth-muscle cells indirectly via the phosphorylation of an associated PI kinase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews W. V., Conn P. M. Measurement of inositol phospholipid metabolites by one-dimensional thin-layer chromatography. Methods Enzymol. 1987;141:156–168. doi: 10.1016/0076-6879(87)41064-1. [DOI] [PubMed] [Google Scholar]
- Casnellie J. E., Greengard P. Guanosine 3':5'-cyclic monophosphate-dependent phosphorylation of endogenous substrate proteins in membranes of mammalian smooth muscle. Proc Natl Acad Sci U S A. 1974 May;71(5):1891–1895. doi: 10.1073/pnas.71.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casnellie J. E., Ives H. E., Jamieson J. D., Greengard P. Cyclic GMP-dependent protein phosphorylation in intact medial tissue and isolated cells from vascular smooth muscle. J Biol Chem. 1980 Apr 25;255(8):3770–3776. [PubMed] [Google Scholar]
- Choquette D., Hakim G., Filoteo A. G., Plishker G. A., Bostwick J. R., Penniston J. T. Regulation of plasma membrane Ca2+ ATPases by lipids of the phosphatidylinositol cycle. Biochem Biophys Res Commun. 1984 Dec 28;125(3):908–915. doi: 10.1016/0006-291x(84)91369-x. [DOI] [PubMed] [Google Scholar]
- Doctrow S. R., Lowenstein J. M. Inhibition of phosphatidylinositol kinase in vascular smooth muscle membranes by adenosine and related compounds. Biochem Pharmacol. 1987 Jul 15;36(14):2255–2262. doi: 10.1016/0006-2952(87)90588-0. [DOI] [PubMed] [Google Scholar]
- Endemann G., Dunn S. N., Cantley L. C. Bovine brain contains two types of phosphatidylinositol kinase. Biochemistry. 1987 Oct 20;26(21):6845–6852. doi: 10.1021/bi00395a039. [DOI] [PubMed] [Google Scholar]
- Enyedi A., Flura M., Sarkadi B., Gardos G., Carafoli E. The maximal velocity and the calcium affinity of the red cell calcium pump may be regulated independently. J Biol Chem. 1987 May 5;262(13):6425–6430. [PubMed] [Google Scholar]
- Furukawa K., Nakamura H. Cyclic GMP regulation of the plasma membrane (Ca2+-Mg2+)ATPase in vascular smooth muscle. J Biochem. 1987 Jan;101(1):287–290. doi: 10.1093/oxfordjournals.jbchem.a121904. [DOI] [PubMed] [Google Scholar]
- Gietzen K., Kolandt J. Large-scale isolation of human erythrocyte Ca2+-transport ATPase. Biochem J. 1982 Oct 1;207(1):155–159. doi: 10.1042/bj2070155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassid A. Atriopeptin II decreases cytosolic free Ca in cultured vascular smooth muscle cells. Am J Physiol. 1986 Nov;251(5 Pt 1):C681–C686. doi: 10.1152/ajpcell.1986.251.5.C681. [DOI] [PubMed] [Google Scholar]
- Hathaway D. R., Konicki M. V., Coolican S. A. Phosphorylation of myosin light chain kinase from vascular smooth muscle by cAMP- and cGMP-dependent protein kinases. J Mol Cell Cardiol. 1985 Sep;17(9):841–850. doi: 10.1016/s0022-2828(85)80098-5. [DOI] [PubMed] [Google Scholar]
- Heil W. G., Landgraf W., Hofmann F. A catalytically active fragment of cGMP-dependent protein kinase. Occupation of its cGMP-binding sites does not affect its phosphotransferase activity. Eur J Biochem. 1987 Oct 1;168(1):117–121. doi: 10.1111/j.1432-1033.1987.tb13395.x. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Flockerzi V. Characterization of phosphorylated and native cGMP-dependent protein kinase. Eur J Biochem. 1983 Feb 15;130(3):599–603. doi: 10.1111/j.1432-1033.1983.tb07191.x. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Gensheimer H. P., Göbel C. cGMP-dependent protein kinase. Autophosphorylation changes the characteristics of binding site 1. Eur J Biochem. 1985 Mar 1;147(2):361–365. doi: 10.1111/j.1432-1033.1985.tb08758.x. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Kadowitz P. J. The pharmacological and physiological role of cyclic GMP in vascular smooth muscle relaxation. Annu Rev Pharmacol Toxicol. 1985;25:171–191. doi: 10.1146/annurev.pa.25.040185.001131. [DOI] [PubMed] [Google Scholar]
- Ives H. E., Casnellie J. E., Greengard P., Jamieson J. D. Subcellular localization of cyclic GMP-dependent protein kinase and its substrates in vascular smooth muscle. J Biol Chem. 1980 Apr 25;255(8):3777–3785. [PubMed] [Google Scholar]
- Kai H., Kanaide H., Matsumoto T., Nakamura M. 8-Bromoguanosine 3':5'-cyclic monophosphate decreases intracellular free calcium concentrations in cultured vascular smooth muscle cells from rat aorta. FEBS Lett. 1987 Sep 14;221(2):284–288. doi: 10.1016/0014-5793(87)80941-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Kanaide H., Nakamura M. Cytosolic-free calcium transients in cultured vascular smooth muscle cells: microfluorometric measurements. Science. 1985 Aug 9;229(4713):553–556. doi: 10.1126/science.3927484. [DOI] [PubMed] [Google Scholar]
- Kosk-Kosicka D., Inesi G. Cooperative calcium binding and calmodulin regulation in the calcium-dependent adenosine triphosphatase purified from the erythrocyte membrane. FEBS Lett. 1985 Sep 9;189(1):67–71. doi: 10.1016/0014-5793(85)80843-7. [DOI] [PubMed] [Google Scholar]
- Kosk-Kosicka D., Scaillet S., Inesi G. The partial reactions in the catalytic cycle of the calcium-dependent adenosine triphosphatase purified from erythrocyte membranes. J Biol Chem. 1986 Mar 5;261(7):3333–3338. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M., Johnson R. M. Possible role of cyclic-GMP-dependent protein kinase in vascular smooth muscle function. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:285–296. [PubMed] [Google Scholar]
- Murad F., Rapoport R. M., Fiscus R. Role of cyclic-GMP in relaxations of vascular smooth muscle. J Cardiovasc Pharmacol. 1985;7 (Suppl 3):S111–S118. [PubMed] [Google Scholar]
- Nelson D. R., Hanahan D. J. Phospholipid and detergent effects on (Ca2+ + Mg2+)ATPase purified from human erythrocytes. Arch Biochem Biophys. 1985 Feb 1;236(2):720–730. doi: 10.1016/0003-9861(85)90678-2. [DOI] [PubMed] [Google Scholar]
- Neyses L., Reinlib L., Carafoli E. Phosphorylation of the Ca2+-pumping ATPase of heart sarcolemma and erythrocyte plasma membrane by the cAMP-dependent protein kinase. J Biol Chem. 1985 Aug 25;260(18):10283–10287. [PubMed] [Google Scholar]
- Niggli V., Adunyah E. S., Carafoli E. Acidic phospholipids, unsaturated fatty acids, and limited proteolysis mimic the effect of calmodulin on the purified erythrocyte Ca2+ - ATPase. J Biol Chem. 1981 Aug 25;256(16):8588–8592. [PubMed] [Google Scholar]
- Niggli V., Adunyah E. S., Penniston J. T., Carafoli E. Purified (Ca2+-Mg2+)-ATPase of the erythrocyte membrane. Reconstitution and effect of calmodulin and phospholipids. J Biol Chem. 1981 Jan 10;256(1):395–401. [PubMed] [Google Scholar]
- Nishikawa M., de Lanerolle P., Lincoln T. M., Adelstein R. S. Phosphorylation of mammalian myosin light chain kinases by the catalytic subunit of cyclic AMP-dependent protein kinase and by cyclic GMP-dependent protein kinase. J Biol Chem. 1984 Jul 10;259(13):8429–8436. [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Parks T. P., Nairn A. C., Greengard P., Jamieson J. D. The cyclic nucleotide-dependent phosphorylation of aortic smooth muscle membrane proteins. Arch Biochem Biophys. 1987 Jun;255(2):361–371. doi: 10.1016/0003-9861(87)90404-8. [DOI] [PubMed] [Google Scholar]
- Popescu L. M., Panoiu C., Hinescu M., Nutu O. The mechanism of cGMP-induced relaxation in vascular smooth muscle. Eur J Pharmacol. 1985 Jan 8;107(3):393–394. doi: 10.1016/0014-2999(85)90269-9. [DOI] [PubMed] [Google Scholar]
- Raeymaekers L., Hofmann F., Casteels R. Cyclic GMP-dependent protein kinase phosphorylates phospholamban in isolated sarcoplasmic reticulum from cardiac and smooth muscle. Biochem J. 1988 May 15;252(1):269–273. doi: 10.1042/bj2520269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeymaekers L., Wuytack F., Casteels R. Subcellular fractionation of pig stomach smooth muscle. A study of the distribution of the (Ca2+ + Mg2+)-ATPase activity in plasmalemma and endoplasmic reticulum. Biochim Biophys Acta. 1985 May 28;815(3):441–454. doi: 10.1016/0005-2736(85)90372-4. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Sodium nitroprusside-induced protein phosphorylation in intact rat aorta is mimicked by 8-bromo cyclic GMP. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6470–6474. doi: 10.1073/pnas.79.21.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashatwar S. S., Cornwell T. L., Lincoln T. M. Effects of 8-bromo-cGMP on Ca2+ levels in vascular smooth muscle cells: possible regulation of Ca2+-ATPase by cGMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5685–5689. doi: 10.1073/pnas.84.16.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R. K., Wang J. H. Preparation and assay of the Ca2+--dependent modulator protein. Adv Cyclic Nucleotide Res. 1979;10:187–198. [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Kuriyama H. Effects of cAMP- and cGMP-dependent protein kinases, and calmodulin on Ca2+ uptake by highly purified sarcolemmal vesicles of vascular smooth muscle. Biochim Biophys Acta. 1984 Jun 13;773(1):83–90. doi: 10.1016/0005-2736(84)90552-2. [DOI] [PubMed] [Google Scholar]
- Takuwa Y., Rasmussen H. Measurement of cytoplasmic free Ca2+ concentration in rabbit aorta using the photoprotein, aequorin. Effect of atrial natriuretic peptide on agonist-induced Ca2+ signal generation. J Clin Invest. 1987 Jul;80(1):248–257. doi: 10.1172/JCI113055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varsanyi M., Tölle H. G., Heilmeyer M. G., Jr, Dawson R. M., Irvine R. F. Activation of sarcoplasmic reticular Ca2+ transport ATPase by phosphorylation of an associated phosphatidylinositol. EMBO J. 1983;2(9):1543–1548. doi: 10.1002/j.1460-2075.1983.tb01621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist J., Wuytack F., De Schutter G., Raeymaekers L., Casteels R. Reconstitution of the purified calmodulin-dependent (Ca2+ + Mg2+)-ATPase from smooth muscle. Cell Calcium. 1984 Jun;5(3):253–263. doi: 10.1016/0143-4160(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Walter U. Cyclic-GMP-regulated enzymes and their possible physiological functions. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:249–258. [PubMed] [Google Scholar]
- Walter U. Distribution of cyclic-GMP-dependent protein kinase in various rat tissues and cell lines determined by a sensitive and specific radioimmunoassay. Eur J Biochem. 1981 Aug;118(2):339–346. doi: 10.1111/j.1432-1033.1981.tb06408.x. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan D., Roberts T., Cantley L. Evidence for two distinct phosphatidylinositol kinases in fibroblasts. Implications for cellular regulation. Biochem J. 1987 Oct 1;247(1):165–174. doi: 10.1042/bj2470165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist R. J. Possible mechanisms underlying the vasorelaxant response to atrial natriuretic factor. Fed Proc. 1986 Aug;45(9):2371–2375. [PubMed] [Google Scholar]
- Wuytack F., De Schutter G., Casteels R. Purification of (Ca2+ + Mg2+)-ATPase from smooth muscle by calmodulin affinity chromatography. FEBS Lett. 1981 Jul 6;129(2):297–300. doi: 10.1016/0014-5793(81)80187-1. [DOI] [PubMed] [Google Scholar]
- Wuytack F., De Schutter G., Verbist J., Casteels R. Antibodies to the calmodulin-binding Ca2+-transport ATPase from smooth muscle. FEBS Lett. 1983 Apr 5;154(1):191–195. doi: 10.1016/0014-5793(83)80901-6. [DOI] [PubMed] [Google Scholar]