Abstract
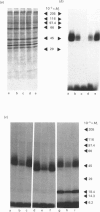
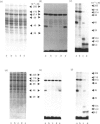
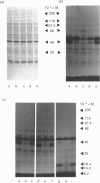
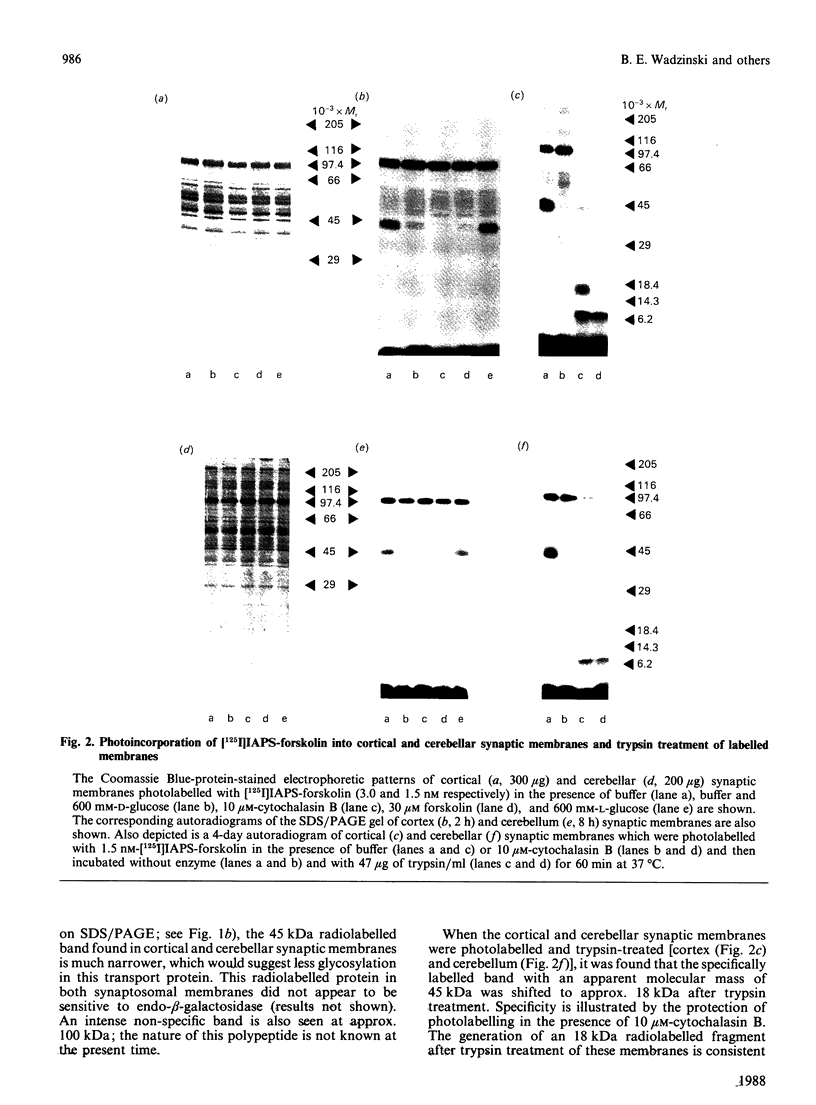
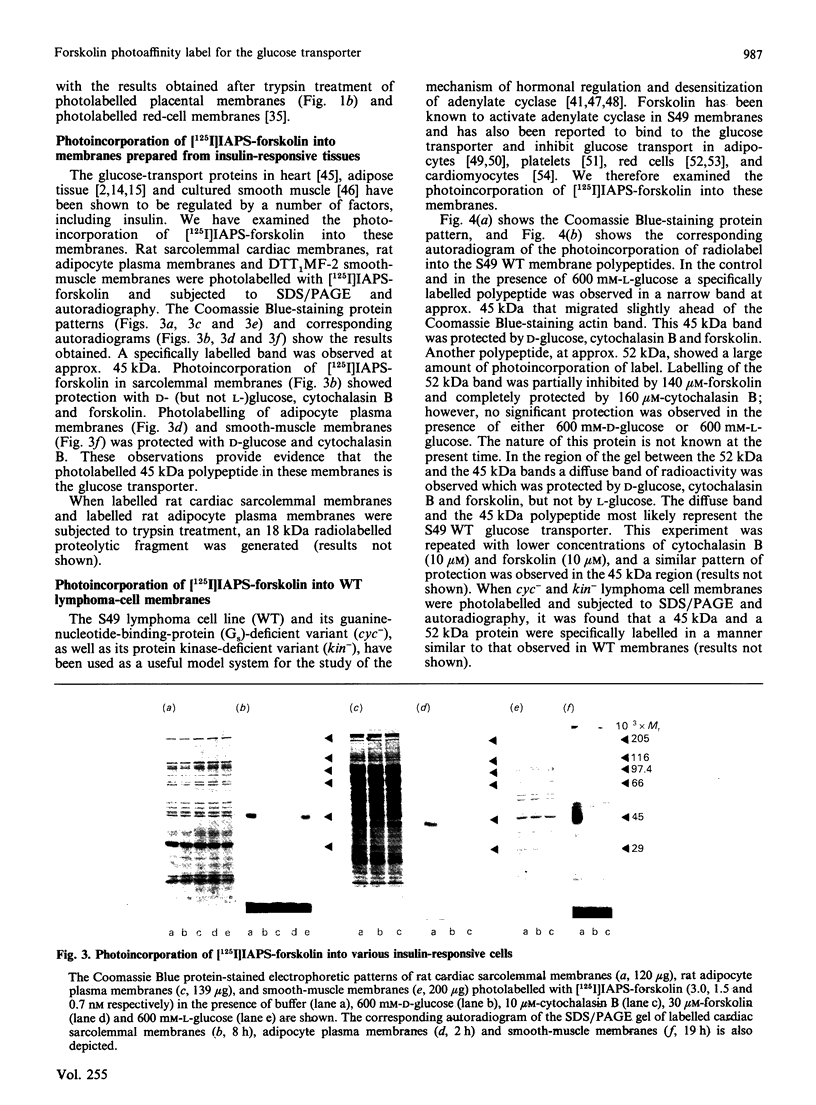
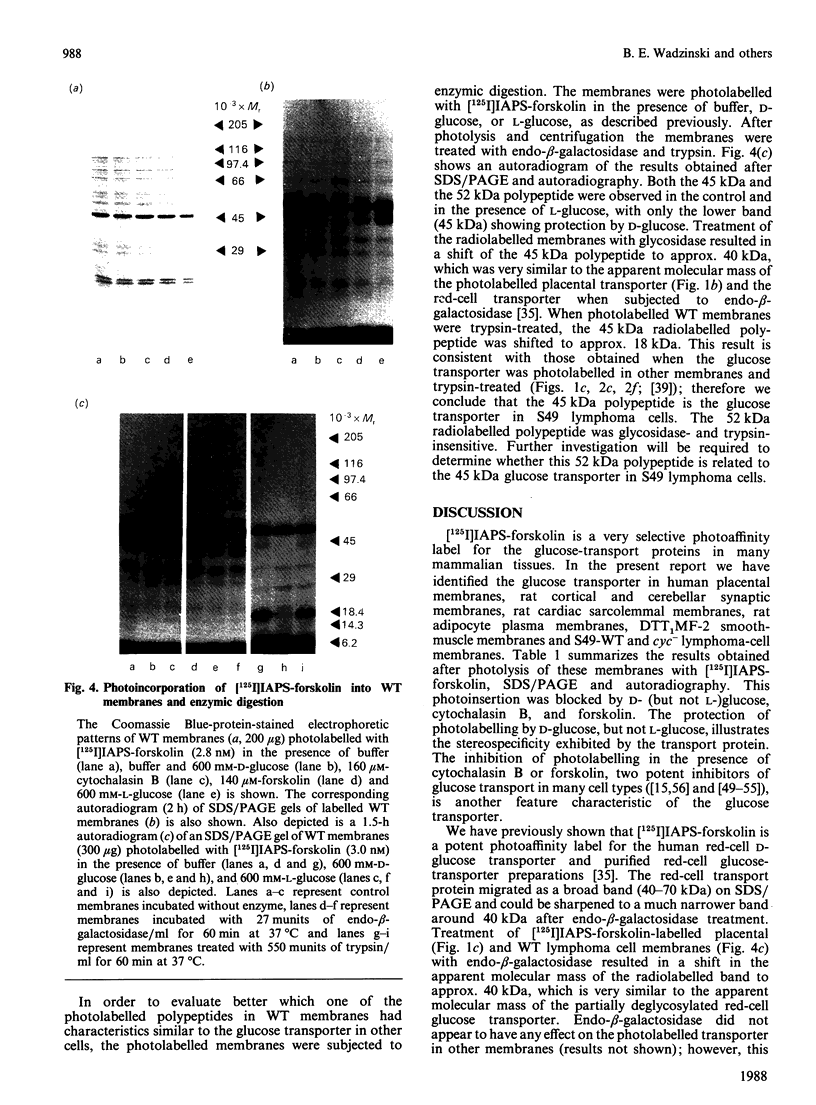
The glucose transporter has been identified in a variety of mammalian cell membranes using a photoactivatable carrier-free radioiodinated derivative of forskolin, 3-[125I]iodo-4-azidophenethylamido-7-O-succinyldeacetylforskoli n ([125I]IAPS-forskolin) at 1-3 nM. The membranes that were photolabelled with [125I]IAPS-forskolin were human placental membranes, rat cortical and cerebellar synaptic membranes, rat cardiac sarcolemmal membranes, rat adipocyte plasma membranes, smooth-muscle membranes, and S49 wild-type (WT) lymphoma-cell membranes. The glucose transporter in plasma membranes prepared from the insulin-responsive rat cardiac sarcolemmal cells, rat adipocytes and smooth-muscle cells were determined to be approx. 45 kDa by SDS/polyacrylamide-gel electrophoresis (PAGE). Photolysis of human placental membranes, rat cortical and cerebellar synaptic membranes, and WT lymphoma membranes with [125I]-IAPS-forskolin, followed by SDS/PAGE, indicated specific derivatization of a broad band (43-55 kDa) in placental membranes and a narrower band (approx. 45 kDa) in synaptic membranes and WT lymphoma membranes. Digestion of the [125I]IAPS-forskolin-labelled placental and WT lymphoma membranes with endo-beta-galactosidase showed a reduction in the apparent molecular mass of the radiolabelled band to approx. 40 kDa. The membranes that were photolabelled with [125I]IAPS-forskolin and trypsin-treated produced a radiolabelled proteolytic fragment with an apparent molecular mass of 18 kDa. [125I]IAPS-forskolin is a highly effective probe for identifying low levels of glucose transporters in mammalian tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin S. A., Cairns M. T., Gardiner R. M., Ruggier R. A D-glucose-sensitive cytochalasin B binding component of cerebral microvessels. J Neurochem. 1985 Aug;45(2):650–652. doi: 10.1111/j.1471-4159.1985.tb04039.x. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns M. T., Elliot D. A., Scudder P. R., Baldwin S. A. Proteolytic and chemical dissection of the human erythrocyte glucose transporter. Biochem J. 1984 Jul 1;221(1):179–188. doi: 10.1042/bj2210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Su C., Pessin J. E., Mora R., Gitomer W., Czech M. P. Photoaffinity labeling of the human erythrocyte D-glucose transporter. J Biol Chem. 1982 May 25;257(10):5419–5425. [PubMed] [Google Scholar]
- Clark R. B. Desensitization of hormonal stimuli coupled to regulation of cyclic AMP levels. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:151–209. [PubMed] [Google Scholar]
- Clark R. B., Friedman J., Johnson J. A., Kunkel M. W. Beta-adrenergic receptor desensitization of wild-type but not cyc lymphoma cells unmasked by submillimolar Mg2+. FASEB J. 1987 Oct;1(4):289–297. doi: 10.1096/fasebj.1.4.2820824. [DOI] [PubMed] [Google Scholar]
- Deziel M. R., Jung C. Y., Rothstein A. The topology of the major band 4.5 protein component of the human erythrocyte membrane: characterization of reactive cysteine residues. Biochim Biophys Acta. 1985 Sep 25;819(1):83–92. doi: 10.1016/0005-2736(85)90198-1. [DOI] [PubMed] [Google Scholar]
- Deziel M. R., Rothstein A. Proteolytic cleavages of cytochalasin B binding components of band 4.5 proteins of the human red blood cell membrane. Biochim Biophys Acta. 1984 Sep 19;776(1):10–20. doi: 10.1016/0005-2736(84)90245-1. [DOI] [PubMed] [Google Scholar]
- Dick A. P., Harik S. I. Distribution of the glucose transporter in the mammalian brain. J Neurochem. 1986 May;46(5):1406–1411. doi: 10.1111/j.1471-4159.1986.tb01755.x. [DOI] [PubMed] [Google Scholar]
- Dick A. P., Harik S. I., Klip A., Walker D. M. Identification and characterization of the glucose transporter of the blood-brain barrier by cytochalasin B binding and immunological reactivity. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7233–7237. doi: 10.1073/pnas.81.22.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese R. V., Standaert M. L., Barnes D. E., Davis J. S., Pollet R. J. Phorbol ester provokes insulin-like effects on glucose transport, amino acid uptake, and pyruvate dehydrogenase activity in BC3H-1 cultured myocytes. Endocrinology. 1985 Jun;116(6):2650–2655. doi: 10.1210/endo-116-6-2650. [DOI] [PubMed] [Google Scholar]
- Giulian G. G., Moss R. L., Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal Biochem. 1983 Mar;129(2):277–287. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- Gorga F. R., Baldwin S. A., Lienhard G. E. The monosaccharide transporter from human erythrocytes is heterogeneously glycosylated. Biochem Biophys Res Commun. 1979 Dec 14;91(3):955–961. doi: 10.1016/0006-291x(79)91972-7. [DOI] [PubMed] [Google Scholar]
- Gurd J. W., Jones L. R., Mahler H. R., Moore W. J. Isolation and partial characterization of rat brain synaptic plasma membranes. J Neurochem. 1974 Feb;22(2):281–290. doi: 10.1111/j.1471-4159.1974.tb11591.x. [DOI] [PubMed] [Google Scholar]
- Hara M., Matsuda Y., Nakagawa H. Molecular characteristics of rat brain glucose transporter: a novel species with Mr 45,000. J Biochem. 1987 Jan;101(1):43–52. doi: 10.1093/oxfordjournals.jbchem.a121906. [DOI] [PubMed] [Google Scholar]
- Holman G. D., Parkar B. A., Midgley P. J. Exofacial photoaffinity labelling of the human erythrocyte sugar transporter. Biochim Biophys Acta. 1986 Feb 13;855(1):115–126. doi: 10.1016/0005-2736(86)90195-1. [DOI] [PubMed] [Google Scholar]
- Ingermann R. L., Bissonnette J. M., Koch P. L. D-Glucose-sensitive and -insensitive cytochalasin B binding proteins from microvillous plasma membranes of human placenta. Identification of the D-glucose transporter. Biochim Biophys Acta. 1983 Apr 21;730(1):57–63. doi: 10.1016/0005-2736(83)90316-4. [DOI] [PubMed] [Google Scholar]
- Ishii T., Tillotson L. G., Isselbacher K. J. Facilitated glucose transporter of human erythrocyte: proteolytic mapping of the [3H]cytochalasin B photoaffinity-labeled transporter polypeptide. Biochim Biophys Acta. 1985 Nov 8;832(1):14–21. doi: 10.1016/0167-4838(85)90169-4. [DOI] [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Farfel Z., Bourne H. R. Genetic analysis of hormone-sensitive adenylate cyclase. Adv Cyclic Nucleotide Res. 1980;13:1–37. [PubMed] [Google Scholar]
- Johnson L. W., Smith C. H. Identification of the glucose transport protein of the microvillous membrane of human placenta by photoaffinity labelling. Biochem Biophys Res Commun. 1982 Nov 30;109(2):408–413. doi: 10.1016/0006-291x(82)91736-3. [DOI] [PubMed] [Google Scholar]
- Jones L. R., Maddock S. W., Besch H. R., Jr Unmasking effect of alamethicin on the (Na+,K+)-ATPase, beta-adrenergic receptor-coupled adenylate cyclase, and cAMP-dependent protein kinase activities of cardiac sarcolemmal vesicles. J Biol Chem. 1980 Oct 25;255(20):9971–9980. [PubMed] [Google Scholar]
- Jones M. N., Nickson J. K. Monosaccharide transport proteins of the human erythrocyte membrane. Biochim Biophys Acta. 1981 Jun 16;650(1):1–20. doi: 10.1016/0304-4157(81)90006-x. [DOI] [PubMed] [Google Scholar]
- Joost H. G., Steinfelder H. J. Forskolin inhibits insulin-stimulated glucose transport in rat adipose cells by a direct interaction with the glucose transporter. Mol Pharmacol. 1987 Mar;31(3):279–283. [PubMed] [Google Scholar]
- Joost H. G., Weber T. M., Cushman S. W., Simpson I. A. Insulin-stimulated glucose transport in rat adipose cells. Modulation of transporter intrinsic activity by isoproterenol and adenosine. J Biol Chem. 1986 Aug 5;261(22):10033–10036. [PubMed] [Google Scholar]
- Kashiwagi A., Huecksteadt T. P., Foley J. E. The regulation of glucose transport by cAMP stimulators via three different mechanisms in rat and human adipocytes. J Biol Chem. 1983 Nov 25;258(22):13685–13692. [PubMed] [Google Scholar]
- Kelley L. K., Smith C. H., King B. F. Isolation and partial characterization of the basal cell membrane of human placental trophoblast. Biochim Biophys Acta. 1983 Sep 21;734(1):91–98. doi: 10.1016/0005-2736(83)90079-2. [DOI] [PubMed] [Google Scholar]
- Kim H. D., Sergeant S., Shukla S. D. Glucose transport in human platelets and its inhibition by forskolin. J Pharmacol Exp Ther. 1986 Mar;236(3):585–589. [PubMed] [Google Scholar]
- Klip A., Walker D., Cohen A., Leung C. Y. Chemical and genetic comparison of the glucose and nucleoside transporters. Biochem Cell Biol. 1986 Nov;64(11):1170–1180. doi: 10.1139/o86-154. [DOI] [PubMed] [Google Scholar]
- Klip A., Walker D., Ransome K. J., Schroer D. W., Lienhard G. E. Identification of the glucose transporter in rat skeletal muscle. Arch Biochem Biophys. 1983 Oct 1;226(1):198–205. doi: 10.1016/0003-9861(83)90285-0. [DOI] [PubMed] [Google Scholar]
- Kuroda M., Honnor R. C., Cushman S. W., Londos C., Simpson I. A. Regulation of insulin-stimulated glucose transport in the isolated rat adipocyte. cAMP-independent effects of lipolytic and antilipolytic agents. J Biol Chem. 1987 Jan 5;262(1):245–253. [PubMed] [Google Scholar]
- Lavis V. R., Lee D. P., Shenolikar S. Evidence that forskolin binds to the glucose transporter of human erythrocytes. J Biol Chem. 1987 Oct 25;262(30):14571–14575. [PubMed] [Google Scholar]
- Lienhard G. E., Kim H. H., Ransome K. J., Gorga J. C. Immunological identification of an insulin-responsive glucose transporter. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1150–1156. doi: 10.1016/0006-291x(82)91090-7. [DOI] [PubMed] [Google Scholar]
- Lindgren C. A., Paulson D. J., Shanahan M. F. Isolated cardiac myocytes. A new cellular model for studying insulin modulation of monosaccharide transport. Biochim Biophys Acta. 1982 Dec 30;721(4):385–393. doi: 10.1016/0167-4889(82)90093-3. [DOI] [PubMed] [Google Scholar]
- May J. M., Horuk R., Olefsky J. M. Photolabeling of the adipocyte hexose carrier with an aryl azide derivative of maltose. Mol Cell Endocrinol. 1987 Feb;49(2-3):181–188. doi: 10.1016/0303-7207(87)90211-5. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Rashidbaigi A., Ruoho A. E., Green D. A., Clark R. B. Photoaffinity labeling of the beta-adrenergic receptor from cultured lymphoma cells with [125I]iodoazidobenzylpindolol: loss of the label with desensitization. Proc Natl Acad Sci U S A. 1983 May;80(10):2849–2853. doi: 10.1073/pnas.80.10.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson F. W., Blevins T. L., Suzuki K., Kono T. An improved method of reconstitution of adipocyte glucose transport activity. Anal Biochem. 1982 May 1;122(1):10–19. doi: 10.1016/0003-2697(82)90244-5. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Maguire M. E., Sturgill T. W., Biltonen R. L., Gilman A. G. Relationship between the beta-adrenergic receptor and adenylate cyclase. J Biol Chem. 1977 Aug 25;252(16):5761–5775. [PubMed] [Google Scholar]
- Sergeant S., Kim H. D. Inhibition of 3-O-methylglucose transport in human erythrocytes by forskolin. J Biol Chem. 1985 Nov 25;260(27):14677–14682. [PubMed] [Google Scholar]
- Shanahan M. F. Characterization of cytochalasin B photoincorporation into human erythrocyte D-glucose transporter and F-actin. Biochemistry. 1983 May 24;22(11):2750–2756. doi: 10.1021/bi00280a024. [DOI] [PubMed] [Google Scholar]
- Shanahan M. F. Cytochalasin B. A natural photoaffinity ligand for labeling the human erythrocyte glucose transporter. J Biol Chem. 1982 Jul 10;257(13):7290–7293. [PubMed] [Google Scholar]
- Shanahan M. F., Czech M. P. Purification and reconstitution of the adipocyte plasma membrane D-glucose transport system. J Biol Chem. 1977 Dec 10;252(23):8341–8343. [PubMed] [Google Scholar]
- Shanahan M. F., D'Artel-Ellis J. Orientation of the glucose transporter in the human erythrocyte membrane. Investigation by in situ proteolytic dissection. J Biol Chem. 1984 Nov 25;259(22):13878–13884. [PubMed] [Google Scholar]
- Shanahan M. F., Edwards B. M., Ruoho A. E. Interactions of insulin, catecholamines and adenosine in the regulation of glucose transport in isolated rat cardiac myocytes. Biochim Biophys Acta. 1986 Jun 16;887(1):121–129. doi: 10.1016/0167-4889(86)90132-1. [DOI] [PubMed] [Google Scholar]
- Shanahan M. F., Morris D. P., Edwards B. M. [3H]forskolin. Direct photoaffinity labeling of the erythrocyte D-glucose transporter. J Biol Chem. 1987 May 5;262(13):5978–5984. [PubMed] [Google Scholar]
- Shanahan M. F., Olson S. A., Weber M. J., Lienhard G. E., Gorga J. C. Photolabeling of glucose-sensitive cytochalasin B binding proteins in erythrocyte, fibroblast and adipocyte membranes. Biochem Biophys Res Commun. 1982 Jul 16;107(1):38–43. doi: 10.1016/0006-291x(82)91666-7. [DOI] [PubMed] [Google Scholar]
- Shanahan M. F., Wadzinski B. E., Lowndes J. M., Ruoho A. E. Photoaffinity labeling of the human erythrocyte monosaccharide transporter with an aryl azide derivative of D-glucose. J Biol Chem. 1985 Sep 15;260(20):10897–10900. [PubMed] [Google Scholar]
- Simpson I. A., Cushman S. W. Hormonal regulation of mammalian glucose transport. Annu Rev Biochem. 1986;55:1059–1089. doi: 10.1146/annurev.bi.55.070186.005211. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Yver D. R., Hissin P. J., Wardzala L. J., Karnieli E., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transporters in the isolated rat adipose cells: characterization of subcellular fractions. Biochim Biophys Acta. 1983 Dec 19;763(4):393–407. doi: 10.1016/0167-4889(83)90101-5. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna R. D., Langdon R. G. Reversible association of cytochalasin B with the human erythrocyte membrane. Inhibition of glucose transport and the stoichiometry of cytochalasin binding. Biochim Biophys Acta. 1973 Oct 11;323(2):207–219. doi: 10.1016/0005-2736(73)90145-4. [DOI] [PubMed] [Google Scholar]
- Wadzinski B. E., Shanahan M. F., Ruoho A. E. Derivatization of the human erythrocyte glucose transporter using a novel forskolin photoaffinity label. J Biol Chem. 1987 Dec 25;262(36):17683–17689. [PubMed] [Google Scholar]
- Wardzala L. J., Cushman S. W., Salans L. B. Mechanism of insulin action on glucose transport in the isolated rat adipose cell. Enhancement of the number of functional transport systems. J Biol Chem. 1978 Nov 25;253(22):8002–8005. [PubMed] [Google Scholar]
- Watanabe T., Smith M. M., Robinson F. W., Kono T. Insulin action on glucose transport in cardiac muscle. J Biol Chem. 1984 Nov 10;259(21):13117–13122. [PubMed] [Google Scholar]
- Weber T. M., Eichholz A. Characterization of a photosensitive glucose derivative. A photoaffinity reagent for the erythrocyte hexose transporter. Biochim Biophys Acta. 1985 Jan 25;812(2):503–511. doi: 10.1016/0005-2736(85)90325-6. [DOI] [PubMed] [Google Scholar]
- Wheeler T. J., Hauck M. A. Reconstitution of the glucose transporter from bovine heart. Biochim Biophys Acta. 1985 Aug 27;818(2):171–182. doi: 10.1016/0005-2736(85)90559-0. [DOI] [PubMed] [Google Scholar]
- Wheeler T. J., Hinkle P. C. The glucose transporter of mammalian cells. Annu Rev Physiol. 1985;47:503–517. doi: 10.1146/annurev.ph.47.030185.002443. [DOI] [PubMed] [Google Scholar]
- Wheeler T. J., Simpson I. A., Sogin D. C., Hinkle P. C., Cushman S. W. Detection of the rat adipose cell glucose transporter with antibody against the human red cell glucose transporter. Biochem Biophys Res Commun. 1982 Mar 15;105(1):89–95. doi: 10.1016/s0006-291x(82)80014-4. [DOI] [PubMed] [Google Scholar]