Abstract
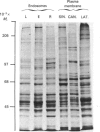
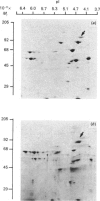
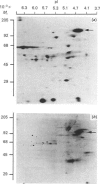
The distribution of calmodulin-binding polypeptides in various rat liver subcellular fractions was investigated. Plasma-membrane, endosome, Golgi and lysosome fractions were prepared by established procedures. The calmodulin-binding polypeptides present in the subcellular fractions were identified by using an overlay technique after transfer from gels to nitrocellulose sheets. Distinctive populations of calmodulin-binding polypeptides were present in all the fractions examined except lysosomes. A major 115 kDa calmodulin-binding polypeptide of pI 4.3 was located to the endosome subfractions, and it emerges as a candidate endosome-specific protein. Partitioning of endosome fractions between aqueous and Triton X-114 phases indicated that the calmodulin-binding polypeptide was hydrophobic. Major calmodulin-binding polypeptides of 140 and 240 kDa and minor polypeptides of 40-60 kDa were present in plasma membranes. The distribution of calmodulin in the various endosome and plasma-membrane fractions was also analysed, and the results indicated that the amounts were high compared with those in the cytosol.
Full text
PDF

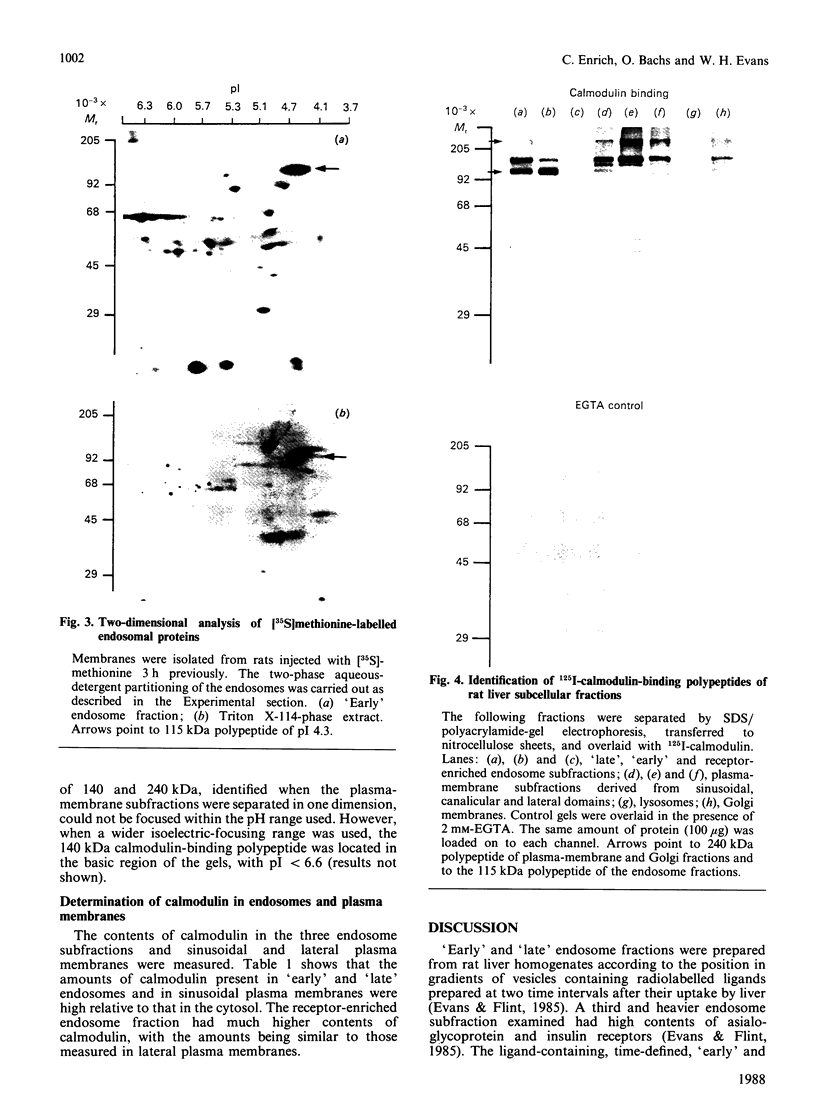
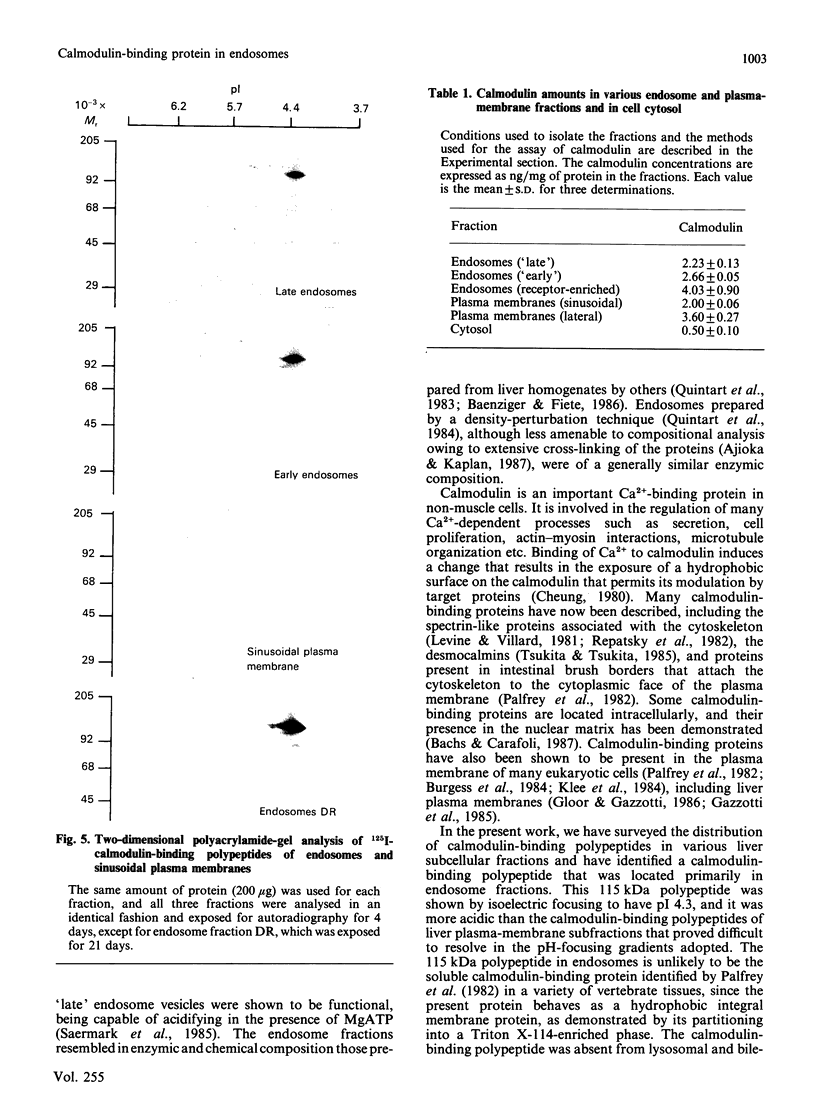


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S. Calmodulin and the regulation of the actin-myosin interaction in smooth muscle and nonmuscle cells. Cell. 1982 Sep;30(2):349–350. doi: 10.1016/0092-8674(82)90232-x. [DOI] [PubMed] [Google Scholar]
- Ajioka R. S., Kaplan J. Characterization of endocytic compartments using the horseradish peroxidase-diaminobenzidine density shift technique. J Cell Biol. 1987 Jan;104(1):77–85. doi: 10.1083/jcb.104.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert K. A., Wu W. C., Nairn A. C., Greengard P. Inhibition by calmodulin of calcium/phospholipid-dependent protein phosphorylation. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3622–3625. doi: 10.1073/pnas.81.12.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein-Gloor M., Gazzotti P. Identification of a fodrin-like protein in rat liver basolateral membranes. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1033–1037. doi: 10.1016/0006-291x(87)91539-7. [DOI] [PubMed] [Google Scholar]
- Bachs O., Carafoli E. Calmodulin and calmodulin-binding proteins in liver cell nuclei. J Biol Chem. 1987 Aug 5;262(22):10786–10790. [PubMed] [Google Scholar]
- Baenziger J. U., Fiete D. Separation of two populations of endocytic vesicles involved in receptor-ligand sorting in rat hepatocytes. J Biol Chem. 1986 Jun 5;261(16):7445–7454. [PubMed] [Google Scholar]
- Beardmore J., Howell K. E., Miller K., Hopkins C. R. Isolation of an endocytic compartment from A431 cells using a density modification procedure employing a receptor-specific monoclonal antibody complexed with colloidal gold. J Cell Sci. 1987 May;87(Pt 4):495–506. doi: 10.1242/jcs.87.4.495. [DOI] [PubMed] [Google Scholar]
- Becker A., Neumeier R., Park C. S., Gossrau R., Reutter W. Identification of a transformation-sensitive 110-kDa plasma membrane glycoprotein of rat hepatocytes. Eur J Cell Biol. 1986 Jan;39(2):417–423. [PubMed] [Google Scholar]
- Belcher J. D., Hamilton R. L., Brady S. E., Hornick C. A., Jaeckle S., Schneider W. J., Havel R. J. Isolation and characterization of three endosomal fractions from the liver of estradiol-treated rats. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6785–6789. doi: 10.1073/pnas.84.19.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Burgess W. H., Watterson D. M., Van Eldik L. J. Identification of calmodulin-binding proteins in chicken embryo fibroblasts. J Cell Biol. 1984 Aug;99(2):550–557. doi: 10.1083/jcb.99.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Chou Y. H., Rebhun L. I. Purification and characterization of a sea urchin egg Ca2+-calmodulin-dependent kinase with myosin light chain phosphorylating activity. J Biol Chem. 1986 Apr 25;261(12):5389–5395. [PubMed] [Google Scholar]
- Evans W. H., Flint N. Subfractionation of hepatic endosomes in Nycodenz gradients and by free-flow electrophoresis. Separation of ligand-transporting and receptor-enriched membranes. Biochem J. 1985 Nov 15;232(1):25–32. doi: 10.1042/bj2320025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Hardison W. G. Phospholipid, cholesterol, polypeptide and glycoprotein composition of hepatic endosome subfractions. Biochem J. 1985 Nov 15;232(1):33–36. doi: 10.1042/bj2320033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H. Preparation of low-density "endosome" and "endosome"-depleted Golgi fractions from rat liver. Methods Enzymol. 1985;109:246–257. doi: 10.1016/0076-6879(85)09090-5. [DOI] [PubMed] [Google Scholar]
- Gazzotti P., Flura M., Gloor M. The association of calmodulin with subcellular fractions isolated from rat liver. Biochem Biophys Res Commun. 1985 Feb 28;127(1):358–365. doi: 10.1016/s0006-291x(85)80167-4. [DOI] [PubMed] [Google Scholar]
- Gloor M., Gazzotti P. The interaction of calmodulin with rat liver plasma membrane. Biochem Biophys Res Commun. 1986 Feb 26;135(1):323–329. doi: 10.1016/0006-291x(86)90980-0. [DOI] [PubMed] [Google Scholar]
- Guerini D., Krebs J., Carafoli E. Stimulation of the purified erythrocyte Ca2+-ATPase by tryptic fragments of calmodulin. J Biol Chem. 1984 Dec 25;259(24):15172–15177. [PubMed] [Google Scholar]
- Hadjiivanova N., Flint N., Evans W. H., Dix C., Cooke B. A. Endocytosis of beta-adrenergic ligands by rat liver. Comparison of beta-adrenergic receptor and adenylate cyclase distribution in endosome and plasma-membrane fractions. Biochem J. 1984 Sep 15;222(3):749–754. doi: 10.1042/bj2220749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay D. G., Khan M. N., Posner B. I., Bergeron J. J. In vivo uptake of insulin into hepatic Golgi fractions: application of the diaminobenzidine-shift protocol. Biochem Biophys Res Commun. 1984 Sep 28;123(3):1144–1148. doi: 10.1016/s0006-291x(84)80252-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine J., Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981 Sep;90(3):631–642. doi: 10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Schmid S., Kern H., Harms E., Male P., Mellman I., Helenius A. Rapid analytical and preparative isolation of functional endosomes by free flow electrophoresis. J Cell Biol. 1987 Apr;104(4):875–886. doi: 10.1083/jcb.104.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S. C., Hubbard A. L. Receptor-mediated endocytosis of asialoglycoproteins by rat hepatocytes: receptor-positive and receptor-negative endosomes. J Cell Biol. 1986 Mar;102(3):932–942. doi: 10.1083/jcb.102.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullock B. M., Hinton R. H., Peppard J. V., Slot J. W., Luzio J. P. The preparative isolation of endosome fractions: a review. Cell Biochem Funct. 1987 Oct;5(4):235–243. doi: 10.1002/cbf.290050402. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Palfrey H. C., Schiebler W., Greengard P. A major calmodulin-binding protein common to various vertebrate tissues. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3780–3784. doi: 10.1073/pnas.79.12.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne M. E., Schworer C. M., Soderling T. R. Purification and characterization of rabbit liver calmodulin-dependent glycogen synthase kinase. J Biol Chem. 1983 Feb 25;258(4):2376–2382. [PubMed] [Google Scholar]
- Petell J. K., Diamond M., Hong W. J., Bujanover Y., Amarri S., Pittschieler K., Doyle D. Isolation and characterization of a Mr = 110,000 glycoprotein localized to the hepatocyte bile canaliculus. J Biol Chem. 1987 Oct 25;262(30):14753–14759. [PubMed] [Google Scholar]
- Quintart J., Courtoy P. J., Baudhuin P. Receptor-mediated endocytosis in rat liver: purification and enzymic characterization of low density organelles involved in uptake of galactose-exposing proteins. J Cell Biol. 1984 Mar;98(3):877–884. doi: 10.1083/jcb.98.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintart J., Courtoy P. J., Limet J. N., Baudhuin P. Galactose-specific endocytosis in rat liver. Biochemical and morphological characterization of a low-density compartment isolated from hepatocytes. Eur J Biochem. 1983 Mar 1;131(1):105–112. doi: 10.1111/j.1432-1033.1983.tb07236.x. [DOI] [PubMed] [Google Scholar]
- Reggio H., Bainton D., Harms E., Coudrier E., Louvard D. Antibodies against lysosomal membranes reveal a 100,000-mol-wt protein that cross-reacts with purified H+,K+ ATPase from gastric mucosa. J Cell Biol. 1984 Oct;99(4 Pt 1):1511–1526. doi: 10.1083/jcb.99.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repasky E. A., Granger B. L., Lazarides E. Widespread occurrence of avian spectrin in nonerythroid cells. Cell. 1982 Jul;29(3):821–833. doi: 10.1016/0092-8674(82)90444-5. [DOI] [PubMed] [Google Scholar]
- Saermark T., Flint N., Evans W. H. Hepatic endosome fractions contain an ATP-driven proton pump. Biochem J. 1985 Jan 1;225(1):51–58. doi: 10.1042/bj2250051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano M., Piñol R., Enrich C., Bachs O. Effect of trifluoperazine on DNA synthesis during liver regeneration. Cell Tissue Kinet. 1985 Sep;18(5):475–481. doi: 10.1111/j.1365-2184.1985.tb00689.x. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Tsukita S. Desmocalmin: a calmodulin-binding high molecular weight protein isolated from desmosomes. J Cell Biol. 1985 Dec;101(6):2070–2080. doi: 10.1083/jcb.101.6.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe P., Krause K. H., Hashimoto S., Zorzato F., Pozzan T., Meldolesi J., Lew D. P. "Calciosome," a cytoplasmic organelle: the inositol 1,4,5-trisphosphate-sensitive Ca2+ store of nonmuscle cells? Proc Natl Acad Sci U S A. 1988 Feb;85(4):1091–1095. doi: 10.1073/pnas.85.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattiaux R., Wattiaux-De Coninck S., Ronveaux-dupal M. F., Dubois F. Isolation of rat liver lysosomes by isopycnic centrifugation in a metrizamide gradient. J Cell Biol. 1978 Aug;78(2):349–368. doi: 10.1083/jcb.78.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileman T., Harding C., Stahl P. Receptor-mediated endocytosis. Biochem J. 1985 Nov 15;232(1):1–14. doi: 10.1042/bj2320001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Functional polarity of the rat hepatocyte surface membrane. Isolation and characterization of plasma-membrane subfractions from the blood-sinusoidal, bile-Canalicular and contiguous surfaces of the hepatocyte. Biochem J. 1975 Feb;146(2):375–388. doi: 10.1042/bj1460375. [DOI] [PMC free article] [PubMed] [Google Scholar]