Abstract
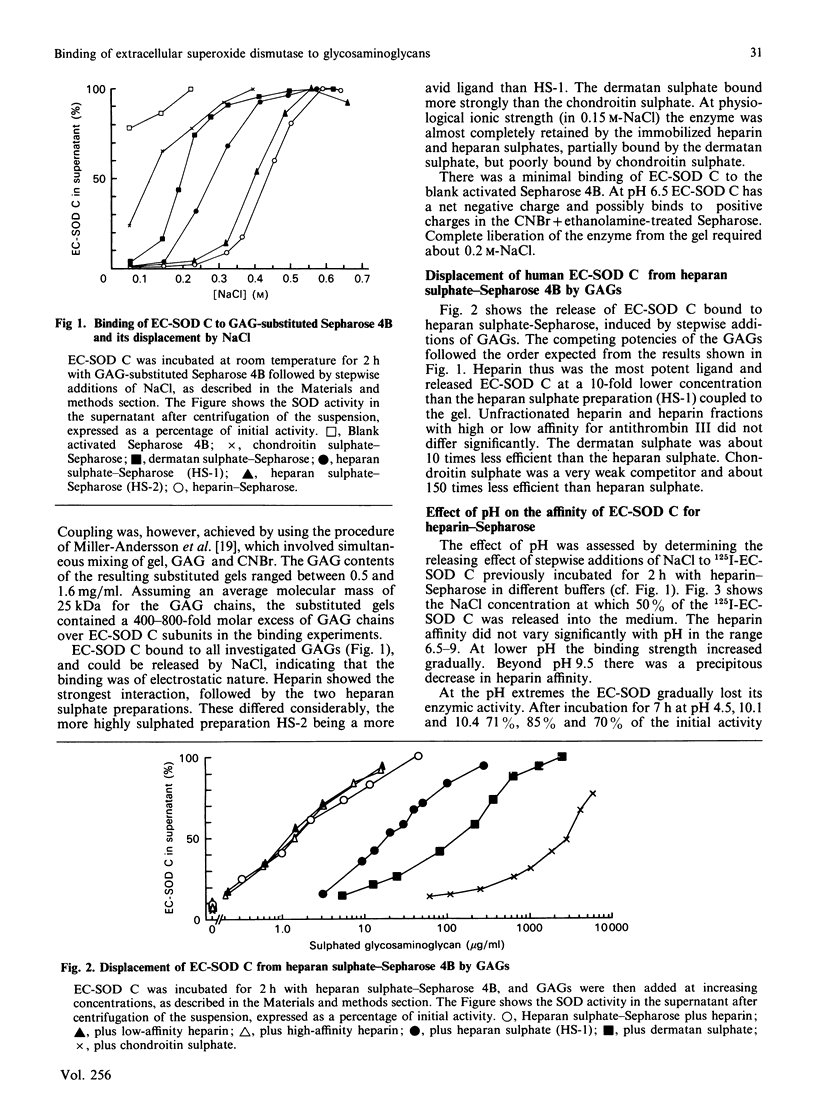
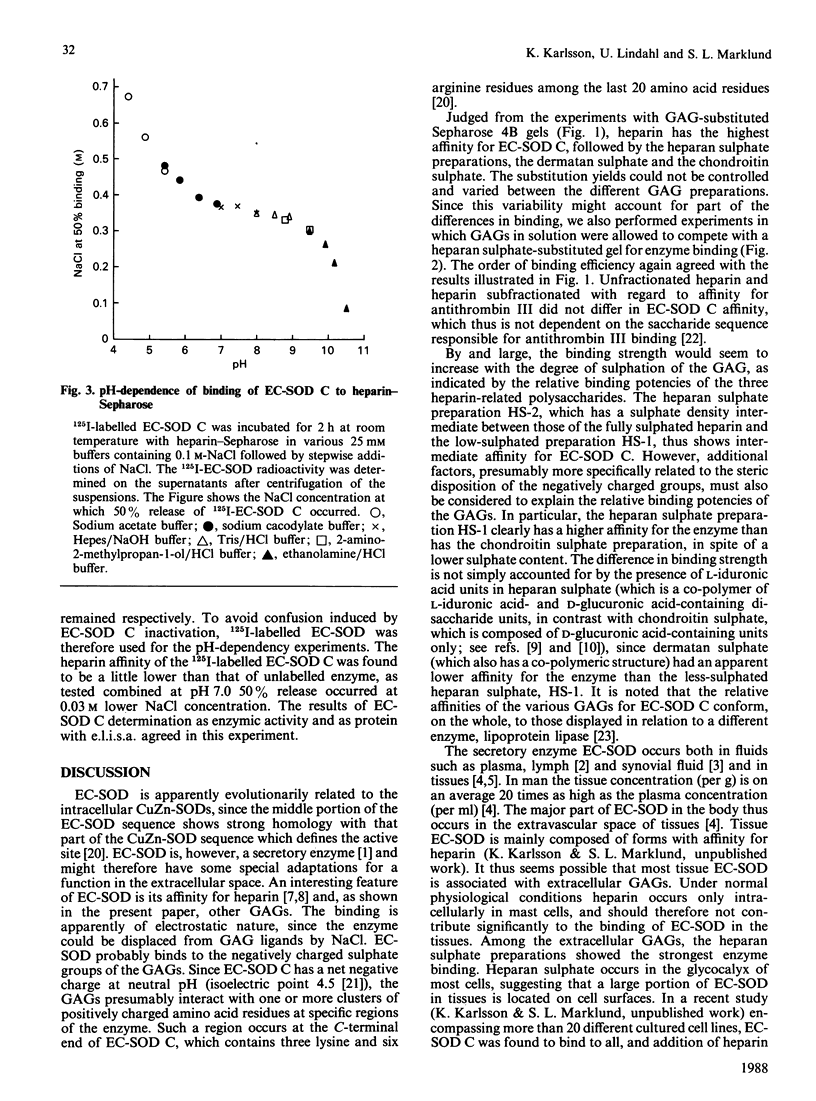
The secretory enzyme extracellular superoxide dismutase (EC-SOD) occurs in at least three forms, which differ with regard to heparin affinity: A lacks affinity, B has intermediate affinity, and C has relatively strong affinity. The affinity of EC-SOD C for various sulphated glycosaminoglycans (GAGs) was assessed (a) by determining the concentration of NaCl required to release the enzyme from GAG-substituted Sepharose 4B and (b) by determining the relative potencies of the GAGs to release EC-SOD C from heparan sulphate-Sepharose 4B. Both methods indicated the same order of affinity. Heparin bound EC-SOD C about 10 times as avidly as the studied heparan sulphate preparation, which in turn was 10 and 150 times as efficient as dermatan sulphate and chondroitin sulphate respectively. Chondroitin sulphate showed weak interaction with EC-SOD C at physiological ionic strength. Heparin subfractions with high or low affinity for antithrombin III were equally efficient. The binding of EC-SOD C to heparin-Sepharose was essentially independent of pH in the range 6.5-9; below pH 6.5 the affinity increased, and beyond pH 9.5 there was a precipitous fall in affinity. The inhibitory effect of NaCl on the binding of EC-SOD C to GAGs indicates that the interaction is of electrostatic nature. EC-SOD C carries a negative net charge at neutral pH, and it is suggested that the binding occurs between the negative charges of the GAG sulphate groups and a structure in the C-terminal end of the enzyme that has a cluster of positive charges. These results are compatible with the notion that heparan sulphate proteoglycans on cell surfaces or in the intercellular matrix may serve to bind EC-SOD C in tissues.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. O., Barrowcliffe T. W., Holmer E., Johnson E. A., Sims G. E. Anticoagulant properties of heparin fractionated by affinity chromatography on matrix-bound antithrombin iii and by gel filtration. Thromb Res. 1976 Dec;9(6):575–583. doi: 10.1016/0049-3848(76)90105-5. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bengtsson G., Olivecrona T., Hök M., Riesenfeld J., Lindahl U. Interaction of lipoprotein lipase with native and modified heparin-like polysaccharides. Biochem J. 1980 Sep 1;189(3):625–633. doi: 10.1042/bj1890625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalmarsson K., Marklund S. L., Engström A., Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hök M., Kjellén L., Johansson S. Cell-surface glycosaminoglycans. Annu Rev Biochem. 1984;53:847–869. doi: 10.1146/annurev.bi.53.070184.004215. [DOI] [PubMed] [Google Scholar]
- Hök M., Riesenfeld J., Lindahl U. N-[3H]Acetyl-labeling, a convenient method for radiolabeling of glycosaminoglycans. Anal Biochem. 1982 Jan 15;119(2):236–245. doi: 10.1016/0003-2697(82)90580-2. [DOI] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Extracellular superoxide dismutase in the vascular system of mammals. Biochem J. 1988 Oct 1;255(1):223–228. [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Heparin-induced release of extracellular superoxide dismutase to human blood plasma. Biochem J. 1987 Feb 15;242(1):55–59. doi: 10.1042/bj2420055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. A., Pejler G., Flynn A. M., Thompson E. A., Lindahl U. Neutralization of heparin-related saccharides by histidine-rich glycoprotein and platelet factor 4. J Biol Chem. 1986 Mar 25;261(9):3980–3986. [PubMed] [Google Scholar]
- Marklund S. L., Bjelle A., Elmqvist L. G. Superoxide dismutase isoenzymes of the synovial fluid in rheumatoid arthritis and in reactive arthritides. Ann Rheum Dis. 1986 Oct;45(10):847–851. doi: 10.1136/ard.45.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984 Sep 15;222(3):649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Extracellular superoxide dismutase in human tissues and human cell lines. J Clin Invest. 1984 Oct;74(4):1398–1403. doi: 10.1172/JCI111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L., Holme E., Hellner L. Superoxide dismutase in extracellular fluids. Clin Chim Acta. 1982 Nov 24;126(1):41–51. doi: 10.1016/0009-8981(82)90360-6. [DOI] [PubMed] [Google Scholar]
- Marklund S. L. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7634–7638. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. Spectrophotometric study of spontaneous disproportionation of superoxide anion radical and sensitive direct assay for superoxide dismutase. J Biol Chem. 1976 Dec 10;251(23):7504–7507. [PubMed] [Google Scholar]
- Nordenman B., Björk I. Influence of ionic strength and pH on the interaction between high-affinity heparin and antithrombin. Biochim Biophys Acta. 1981 Feb 5;672(3):227–238. doi: 10.1016/0304-4165(81)90289-0. [DOI] [PubMed] [Google Scholar]
- Ohman M., Marklund S. L. Plasma extracellular superoxide dismutase and erythrocyte Cu,Zn-containing superoxide dismutase in alcoholics treated with disulfiram. Clin Sci (Lond) 1986 Apr;70(4):365–369. doi: 10.1042/cs0700365. [DOI] [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Simpson P. J., Lucchesi B. R. Free radicals and myocardial ischemia and reperfusion injury. J Lab Clin Med. 1987 Jul;110(1):13–30. [PubMed] [Google Scholar]
- Tibell L., Hjalmarsson K., Edlund T., Skogman G., Engström A., Marklund S. L. Expression of human extracellular superoxide dismutase in Chinese hamster ovary cells and characterization of the product. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6634–6638. doi: 10.1073/pnas.84.19.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]


