Abstract
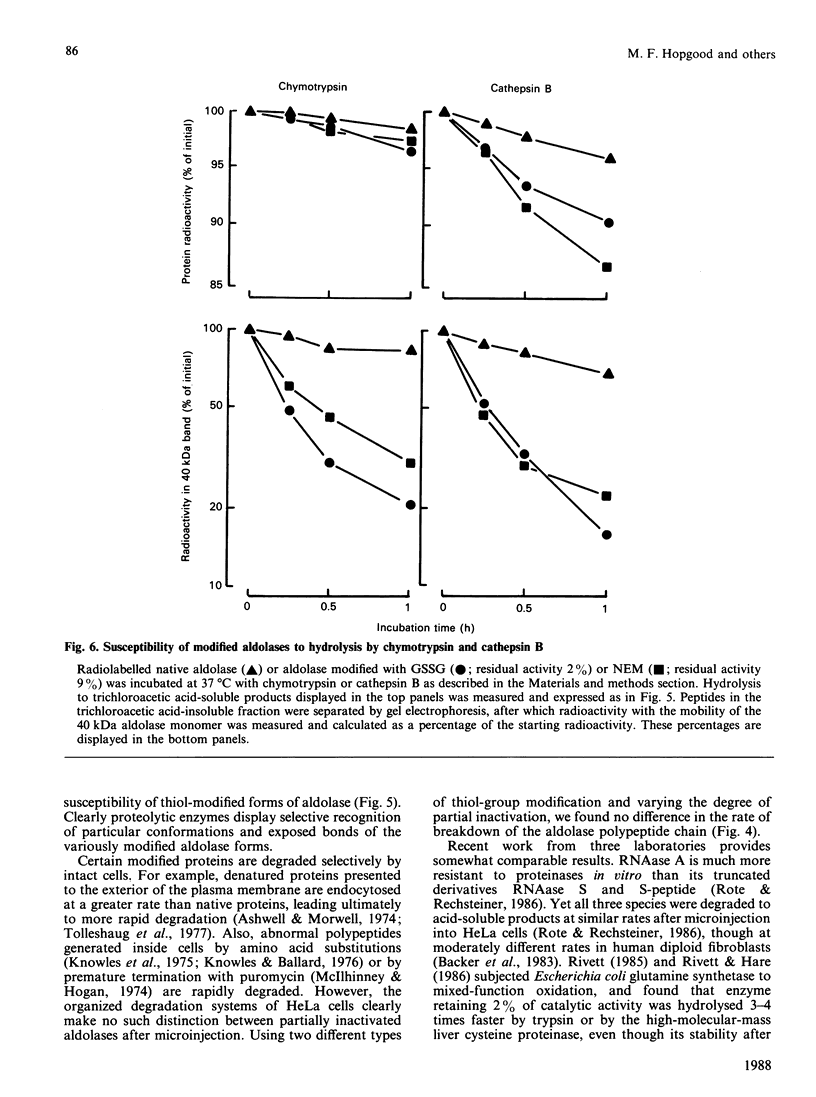
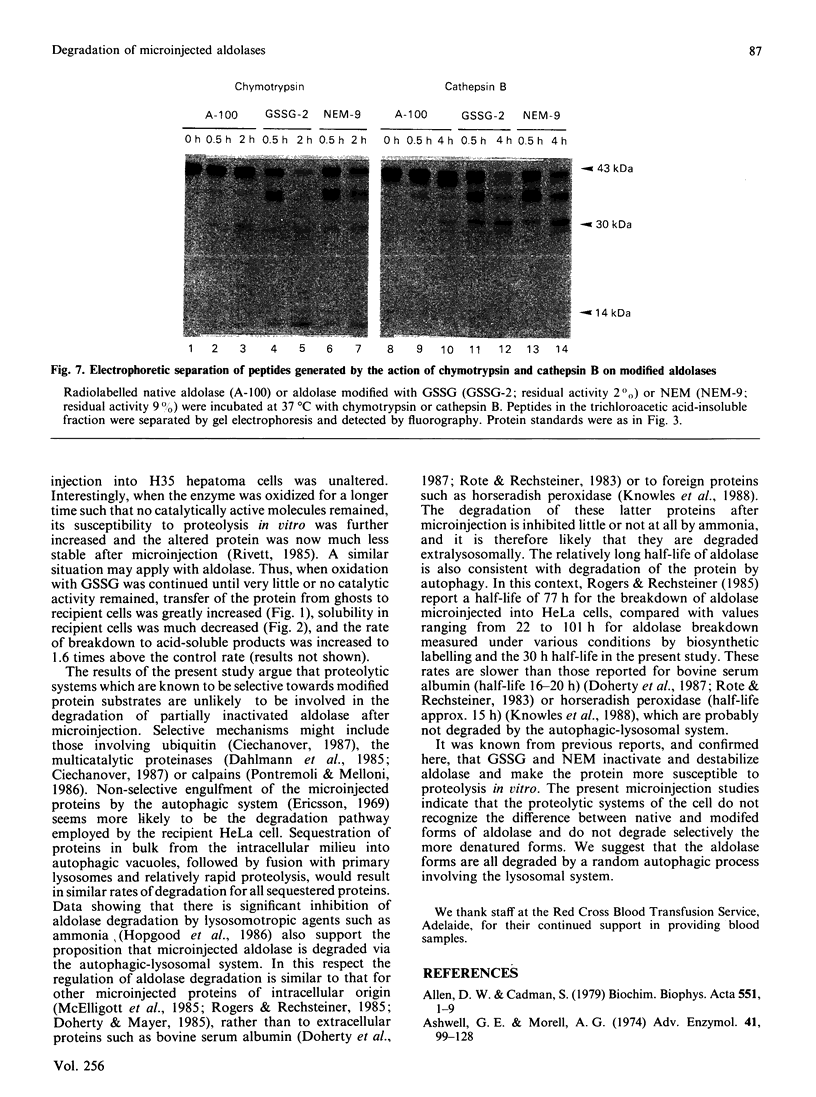
The uptake and degradation of radiolabelled rabbit muscle fructose-bisphosphate aldolase (EC 4.1.2.13) was studied in HeLa cells microinjected by the erythrocyte ghost fusion system. Labelled aldolase was progressively modified by treatment with GSSG or N-ethylmaleimide (NEM) before microinjection to determine whether these agents, which inactivate and destabilize the enzyme in vitro, affect the half-life of the enzyme in vivo. Increasing exposure of aldolase to GSSG or NEM before microinjection increased the extent of aldolase transfer into the HeLa cells and decreased the proportion of the protein that could be extracted from the cells after water lysis. Some degradation of the GSSG- and NEM-inactivated aldolases was observed in the ghosts before microinjection; thus a family of radiolabelled proteins was microinjected in these experiments. In spite of the above differences, the 40 kDa subunit of each aldolase form was degraded with a half-life of 30 h in the HeLa cells. In contrast, the progressively modified forms of aldolase were increasingly susceptible to proteolytic action in vitro by chymotrypsin or by cathepsin B and in ghosts. These studies indicate that the rate of aldolase degradation in cells is not determined by attack by cellular proteinases that recognize vulnerable protein substrates; the results are most easily explained by a random autophagic process involving the lysosomal system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. W., Cadman S. Calcium-induced erythrocyte membrane changes. The role of adsorption of cytosol proteins and proteases. Biochim Biophys Acta. 1979 Feb 20;551(1):1–9. doi: 10.1016/0005-2736(79)90348-1. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- BLOSTEIN R., RUTTER W. J. COMPARATIVE STUDIES OF LIVER AND MUSCLE ALDOLASE. II. IMMUNOCHEMICAL AND CHROMATOGRAPHIC DIFFERENTIATION. J Biol Chem. 1963 Oct;238:3280–3285. [PubMed] [Google Scholar]
- Backer J. M., Bourret L., Dice J. F. Regulation of catabolism of microinjected ribonuclease A requires the amino-terminal 20 amino acids. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2166–2170. doi: 10.1073/pnas.80.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J. Intracellular protein degradation. Essays Biochem. 1977;13:1–37. [PubMed] [Google Scholar]
- Beynon R. J., Bond J. S. Catabolism of intracellular protein: molecular aspects. Am J Physiol. 1986 Aug;251(2 Pt 1):C141–C152. doi: 10.1152/ajpcell.1986.251.2.C141. [DOI] [PubMed] [Google Scholar]
- Bond J. S., Offermann M. K. Initial events in the degradation of soluble cellular enzymes: factors affecting the stability and proteolytic susceptibility of fructose-1,6-bisphosphate aldolase. Acta Biol Med Ger. 1981;40(10-11):1365–1374. [PubMed] [Google Scholar]
- Chandler C. S., Ballard F. J. Distribution and degradation of biotin-containing carboxylases in human cell lines. Biochem J. 1985 Dec 1;232(2):385–393. doi: 10.1042/bj2320385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Finley D., Varshavsky A. The ubiquitin-mediated proteolytic pathway and mechanisms of energy-dependent intracellular protein degradation. J Cell Biochem. 1984;24(1):27–53. doi: 10.1002/jcb.240240104. [DOI] [PubMed] [Google Scholar]
- Dahlmann B., Kuehn L., Rutschmann M., Reinauer H. Purification and characterization of a multicatalytic high-molecular-mass proteinase from rat skeletal muscle. Biochem J. 1985 May 15;228(1):161–170. doi: 10.1042/bj2280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty F. J., Mayer R. J. Degradation of erythrocyte-microinjected and scrape-loaded homologous cytosolic proteins by 3T3-L1 cells. Biochem J. 1985 Mar 15;226(3):685–695. doi: 10.1042/bj2260685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty F. J., Wassell J. A., Mayer R. J. A putative protein-sequestration site involving intermediate filaments for protein degradation by autophagy. Studies with microinjected purified glycolytic enzymes in 3T3-L1 cells. Biochem J. 1987 Feb 1;241(3):793–800. doi: 10.1042/bj2410793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan J. M., Waxman L., Goldberg A. L. Red blood cells contain a pathway for the degradation of oxidant-damaged hemoglobin that does not require ATP or ubiquitin. J Biol Chem. 1986 May 5;261(13):5705–5713. [PubMed] [Google Scholar]
- Francis G. L., Ballard F. J. Enzyme inactivation via disulphide-thiol exchange as catalysed by a rat liver membrane protein. Biochem J. 1980 Feb 15;186(2):581–590. doi: 10.1042/bj1860581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Boches F. S. Oxidized proteins in erythrocytes are rapidly degraded by the adenosine triphosphate-dependent proteolytic system. Science. 1982 Feb 26;215(4536):1107–1109. doi: 10.1126/science.7038874. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. doi: 10.1146/annurev.bi.51.070182.002003. [DOI] [PubMed] [Google Scholar]
- Hopgood M. F., Knowles S. E., Ballard F. J. Degradation of microinjected glycolytic enzymes in L6 myoblasts. Biomed Biochim Acta. 1986;45(11-12):1603–1610. [PubMed] [Google Scholar]
- KOWAL J., CREMONA T., HORECKER B. L. THE MECHANISM OF ACTION OF ALDOLASES. IX. NATURE OF THE GROUPS REACTIVE WITH CHLORODINITROBENZENE. J Biol Chem. 1965 Jun;240:2485–2490. [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles S. E., Gunn J. M., Hanson R. W., Ballard F. J. Increased degradation rates of protein synthesized in hepatoma cells in the presence of amino acid analogues. Biochem J. 1975 Mar;146(3):595–600. doi: 10.1042/bj1460595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles S. E., Hopgood M. F., Ballard F. J. Degradation of horseradish peroxidase after microinjection into mammalian cells. Exp Cell Res. 1988 Jan;174(1):266–278. doi: 10.1016/0014-4827(88)90160-7. [DOI] [PubMed] [Google Scholar]
- Levine R. L., Oliver C. N., Fulks R. M., Stadtman E. R. Turnover of bacterial glutamine synthetase: oxidative inactivation precedes proteolysis. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2120–2124. doi: 10.1073/pnas.78.4.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. L. Oxidative modification of glutamine synthetase. I. Inactivation is due to loss of one histidine residue. J Biol Chem. 1983 Oct 10;258(19):11823–11827. [PubMed] [Google Scholar]
- Mayer R. J., Doherty F. Intracellular protein catabolism: state of the art. FEBS Lett. 1986 Mar 31;198(2):181–193. doi: 10.1016/0014-5793(86)80403-3. [DOI] [PubMed] [Google Scholar]
- McElligott M. A., Miao P., Dice J. F. Lysosomal degradation of ribonuclease A and ribonuclease S-protein microinjected into the cytosol of human fibroblasts. J Biol Chem. 1985 Oct 5;260(22):11986–11993. [PubMed] [Google Scholar]
- McIlhinney A., Hogan B. L. Rapid degradation of puromycyl peptides in hepatoma cells and reticulocytes. FEBS Lett. 1974 Apr 1;40(2):297–301. doi: 10.1016/0014-5793(74)80248-6. [DOI] [PubMed] [Google Scholar]
- Midelfort C. F., Mehler A. H. A chymotrypsin-catalyzed modification of rabbit muscle aldolase. J Biol Chem. 1972 Jun 10;247(11):3618–3621. [PubMed] [Google Scholar]
- Murakami T., Suzuki Y., Murachi T. An acid protease in human erythrocytes and its localization in the inner membrane. Eur J Biochem. 1979 May 15;96(2):221–227. doi: 10.1111/j.1432-1033.1979.tb13032.x. [DOI] [PubMed] [Google Scholar]
- Neff N. T., Bourret L., Miao P., Dice J. F. Degradation of proteins microinjected into IMR-90 human diploid fibroblasts. J Cell Biol. 1981 Oct;91(1):184–194. doi: 10.1083/jcb.91.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland P. A., Dice J. F. Red blood cell-mediated microinjection: methodological considerations. Anal Biochem. 1985 Oct;150(1):214–220. doi: 10.1016/0003-2697(85)90461-0. [DOI] [PubMed] [Google Scholar]
- Offermann M. K., Chlebowski J. F., Bond J. S. Action of cathepsin D on fructose-1,6-bisphosphate aldolase. Biochem J. 1983 Jun 1;211(3):529–534. doi: 10.1042/bj2110529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermann M. K., McKay M. J., Marsh M. W., Bond J. S. Glutathione disulfide inactivates, destabilizes, and enhances proteolytic susceptibility of fructose-1,6-bisphosphate aldolase. J Biol Chem. 1984 Jul 25;259(14):8886–8891. [PubMed] [Google Scholar]
- Pontremoli S., Melloni E. Extralysosomal protein degradation. Annu Rev Biochem. 1986;55:455–481. doi: 10.1146/annurev.bi.55.070186.002323. [DOI] [PubMed] [Google Scholar]
- Rivett A. J. Purification of a liver alkaline protease which degrades oxidatively modified glutamine synthetase. Characterization as a high molecular weight cysteine proteinase. J Biol Chem. 1985 Oct 15;260(23):12600–12606. [PubMed] [Google Scholar]
- Robinson A. B., McKerrow J. H., Cary P. Controlled deamidation of peptides and proteins: an experimental hazard and a possible biological timer. Proc Natl Acad Sci U S A. 1970 Jul;66(3):753–757. doi: 10.1073/pnas.66.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rote K. V., Rechsteiner M. Degradation of microinjected proteins: effects of lysosomotropic agents and inhibitors of autophagy. J Cell Physiol. 1983 Jul;116(1):103–110. doi: 10.1002/jcp.1041160116. [DOI] [PubMed] [Google Scholar]
- Rote K. V., Rechsteiner M. Degradation of proteins microinjected into HeLa cells. The role of substrate flexibility. J Biol Chem. 1986 Nov 25;261(33):15430–15436. [PubMed] [Google Scholar]
- Tack B. F., Dean J., Eilat D., Lorenz P. E., Schechter A. N. Tritium labeling of proteins to high specific radioactivity by reduction methylation. J Biol Chem. 1980 Sep 25;255(18):8842–8847. [PubMed] [Google Scholar]
- Tolleshaug H., Berg T., Nilsson M., Norum K. R. Uptake and degradation of 125I-labelled asialo-fetuin by isolated rat hepatocytes. Biochim Biophys Acta. 1977 Aug 25;499(1):73–84. doi: 10.1016/0304-4165(77)90230-6. [DOI] [PubMed] [Google Scholar]
- Tökés Z. A., Chambers S. M. Proteolytic activity associated with human erythrocyte membranes. Self-digestion of isolated human erythrocyte membranes. Biochim Biophys Acta. 1975 May 6;389(2):325–338. doi: 10.1016/0005-2736(75)90325-9. [DOI] [PubMed] [Google Scholar]
- Yamaizumi M., Uchida T., Okada Y., Furusawa M. Neutralization of diphtheria toxin in living cells by microinjection of antifragment A contained within resealed erythrocyte ghosts. Cell. 1978 Feb;13(2):227–232. doi: 10.1016/0092-8674(78)90191-5. [DOI] [PubMed] [Google Scholar]