Abstract
The prognostic significance of the six-minute walk distance for lower extremity events in people with peripheral artery disease (PAD) is unknown. This longitudinal study assessed whether a poorer six-minute walk distance at baseline was associated with higher rates of subsequent lower extremity atherosclerotic disease events in PAD. A total of 369 patients (mean age 69.4 ± 10.0 years; mean ankle–brachial index (ABI) 0.67 ± 0.17; 31% women; 30% black individuals) from Chicago-area medical centers with PAD were enrolled. Participants underwent baseline six-minute walk testing and returned for annual study visits. Lower extremity events consisted of one or more of the following: ABI decline greater than 15% or medical record adjudicated lower extremity revascularization, critical limb ischemia, or amputation. At a mean follow-up of 33.3 months, lower extremity events occurred in 66/123 (53.7%) people in the first (worst) tertile of six-minute walk performance, 55/124 (44.4%) in the second tertile, and 56/122 (45.9%) in the third (best) tertile. After adjusting for age, sex, race, ABI, comorbidities, and other confounders, participants in the first (worst) tertile of six-minute walk distance at baseline had higher rates of lower extremity events during follow-up, compared to those in the best tertile at baseline (HR = 1.74, 95% CI 1.17–2.60, p = 0.0067). Among people with PAD, a poorer six-minute walk distance was associated with higher rates of subsequent lower extremity PAD-related events after adjusting for confounders. Further study is needed to determine whether interventions that improve six-minute walk distance can reduce lower extremity adverse events in people with PAD.
Keywords: amputation, critical limb ischemia (CLI), peripheral artery disease (PAD), six-minute walk
Introduction
The six-minute walk test is a simple and inexpensive test of walking endurance in people with lower extremity peripheral artery disease (PAD) that is commonly used as an outcome measure in studies of people with PAD.1–8 The six-minute walk test can easily be incorporated into clinical practice. Poorer six-minute walk performance has been associated with increased cardiovascular event rates, all-cause mortality, and mobility loss among people with PAD.2–4 However, the association of six-minute walk performance with subsequent lower extremity PAD-related events is unknown.
This study examined the association between baseline six-minute walk distance and subsequent progression of PAD (i.e. lower extremity events). Lower extremity events were defined as one or more of the following during follow-up: clinically meaningful ankle–brachial index (ABI) decline, lower extremity revascularization, critical limb ischemia, or amputation. The study hypothesis was that a shorter baseline six-minute walk distance would be independently associated with higher rates of lower extremity events among people with PAD. To assess whether other measures of functional performance were also associated with increased rates of lower extremity events among people with PAD, we also studied associations of the Walking Impairment Questionnaire (WIQ) scores and physical activity level with subsequent lower extremity events.
Methods
The Walking and Leg Circulation Study (WALCS) III was a longitudinal observational study of participants with PAD, designed to examine the association of magnetic resonance imaging (MRI)-measured atherosclerotic plaque in the superficial femoral artery with functional impairment and decline in people with PAD.9 After enrollment and baseline measurements, participants underwent annual assessment. The institutional review board at participating sites approved the protocol. All participants provided written, informed consent.
Recruitment
Participants with PAD were identified from lists of consecutive patients with PAD from noninvasive vascular laboratories at Northwestern Memorial Hospital, Jesse Brown Veterans Administration, Rush Medical Center, and Mt Sinai Hospital in Chicago. Consecutive patients with PAD were also identified from vascular surgery, cardiology, endocrinology, general medicine, and geriatrics at the Northwestern Medical Faculty Foundation and from the vascular surgery practice at the Jesse Brown VA. Participants were enrolled between October 2007 and December 2009. Follow-up was completed September 10, 2014.
Inclusion and exclusion criteria
The inclusion criterion was an ABI < 1.00.9 Current clinical practice guidelines state that normal ABI values are 1.00–1.40.10 Exclusion criteria have been reported9 and are summarized here: potential participants with dementia and those with a mini-mental status examination (MMSE) score < 23,11 nursing home residents, wheelchair-bound patients, patients with foot or leg amputations, patients who had undergone major surgery in the past 3 months or had a contraindication to MRI, patients who required oxygen during ambulation, stopped the six-minute walk test due to dyspnea, or had severe knee osteoarthritis at baseline, and non-English-speaking participants.
ABI measurement
Systolic blood pressures were measured using a hand-held Doppler probe (Pocket-Dop II; Nicolet Vascular, Golden, CO, USA) after the participant had rested supine for 5 minutes.12 Pressures were measured in the right brachial, dorsalis pedis, and posterior tibial arteries and left dorsalis pedis, posterior tibial, and brachial arteries. Pressures were then repeated in reverse order. The ABI was calculated for each leg by dividing the average of the dorsalis pedis and posterior tibial artery pressures in each leg by the average of the four brachial pressures.13
Six-minute walk test
The six-minute walk test was performed following a standardized protocol.2–4 Participants were asked to walk back and forth down a 30.48-m (100 ft) hallway, covering as much ground as possible for 6 minutes. Study coordinators used a script to provide instructions during the test, and at each minute a standardized phrase of encouragement was given by the coordinator, according to the script. Participants were allowed to rest, but timing continued during rest periods. The total distance walked during the 6 minutes was recorded.
Baseline comorbidities
Comorbidities were identified using algorithms developed for the Women’s Health and Aging Study.14 The algorithm combines data from patient report, medical record review, medications, laboratory values, and a primary care physician questionnaire. Comorbidities assessed included diabetes mellitus, angina, myocardial infarction (MI), heart failure, stroke, pulmonary disease, and cancer. Hypertension was defined as patient report of physician-diagnosed hypertension or physician designation of the presence of hypertension on the primary care physician questionnaire.
Walking Impairment Questionnaire
Participants completed the WIQ, which consisted of three domains: walking distance, walking speed, and stair climbing.15 For the distance score, participants ranked their difficulty walking distances ranging from indoors in their home to up to 1500 feet. For the walking speed score, participants ranked their difficulty walking one block at specific speeds, ranging from slowly to jogging. For the stair climbing score, participants ranked the degree of difficulty climbing one to three flights of stairs. A score was calculated, for each domain, ranging from 0 to 100, with 0 representing inability to perform any of the tasks within that domain and 100 indicating no difficulty with any of the tasks within that domain.15
Physical activity measurement
Participants wore a vertical accelerometer (Caltrac; Muscle Dynamics Fitness Network Inc., Torrance, CA, USA) for 7 days to continuously measure their physical activity.16–20 Using validated methods, the accelerometer was programmed identically for all participants, allowing equivalent comparisons of physical activity, regardless of individual characteristics such as sex, age, and weight. This method is a validated measure of physical activity in people with and without PAD.16–20
Other measures
Height and weight were measured at baseline. Body mass index (BMI) was calculated as weight in kilograms divided by meters squared. Cigarette smoking was determined by self-report. Participants brought their medication bottles or a medication list to their study visits and all medications were recorded. Use of a statin medication was determined by the principal investigator (MMM), who was blinded to participant characteristics. The homogenous direct method from Roche Diagnostics (Indianapolis, IN, USA) was used to measure low density lipoprotein cholesterol (LDL-C).21 High density lipoprotein cholesterol (HDL-C) was measured by direct enzymatic colorimetric assay.22
Measurement and adjudication of lower extremity events during follow-up
After baseline testing, participants returned to the medical center for study visits for 1-year, 2-year, and 4-year follow-ups. At each follow-up visit, participants were interviewed to identify new hospitalizations and lower extremity procedures since the last assessment. At 3 years after baseline, participants were telephoned and asked about any hospitalizations since the prior visit. Medical records were obtained for all hospitalizations.
A lower extremity event during follow-up was defined as one or more of the following: decline in the ABI by 15% or greater, medical record documented hospitalization for lower extremity critical limb ischemia or amputation, or medical record documented lower extremity revascularization. Although ABI declines of 0.15 have been used to define meaningful decline,23–25 people with lower ABI values are less likely to have an ABI decline ≥ 0.15.23–25 Furthermore, in severe PAD, an ABI decline ≥ 0.15 represents a more substantial decline relative to baseline than an ABI decline of 0.15 in mild PAD. Therefore, a meaningful ABI decline was defined as a decline of ≥ 15%. For critical limb ischemia, amputation, and lower extremity revascularization, two investigators independently reviewed the medical record for documentation of hospitalization for critical limb ischemia, amputation, or lower extremity revascularization. Data were recorded on a medical record abstraction form by two independent investigators not aware of baseline six-minute performance. When the reviewers did not agree, they reviewed the medical records together and discussed the outcome until consensus was reached.
Statistical analyses
Baseline characteristics for PAD participants with a lower extremity event versus those without an event were compared using chi-squared tests for categorical variables and general linear models for continuous variables. The six-minute walk distance at baseline was categorized into tertiles. The cause-specific cumulative incidence rate of lower extremity events was estimated in different tertiles of six-minute walk performance, treating death from any cause as a competing risk. Log-rank tests were used to compare the cause-specific incidence rate of lower extremity events across tertiles of six-minute walk performance. Participants were censored due to loss to follow-up. Analyses were repeated for each individual lower extremity outcome (ABI decline, critical limb ischemia or amputation, or lower extremity revascularization). Cox proportional hazards regression was performed to compare specific hazard rates for lower extremity events across baseline tertiles of six-minute walk performance, adjusting for age, sex, race (black vs others), BMI, and comorbidities (current/former smoking, hypertension, diabetes, log-transformed LDL/HDL, statin use). Analyses were repeated with additional adjustment for baseline ABI and performed for the combined lower extremity event outcome and for each individual outcome. Regression analyses were repeated, comparing event rates across baseline tertiles of the WIQ distance, speed, and stair climbing scores and across physical activity tertiles. Analyses were repeated to determine whether stopping during the six-minute walk test, time to onset of leg symptoms during the six-minute walk, and the amount of time rested during the six-minute walk test were associated with lower extremity outcomes.
Statistical analyses were performed using SAS software, Version 9.4 (SAS Institute Inc., Cary, NC, USA). Results were considered statistically significant, if the two-sided p-value was below 0.05.
Results
Among 473 PAD participants in the WALCS III cohort, 48 dropped out or died before the first follow-up visit. Of the remaining 425, 56 were missing at least one baseline variable and were excluded. Four individuals missing ABI data at follow-up were excluded from the analyses of ABI decline but included in all other analyses. Compared to WALCS III participants included in the analyses, those excluded had a higher proportion of females (50% vs 31%, p < 0.01), African Americans (42% vs 30%, p = 0.02), and people with a history of cancer (24% vs 15%). Compared to participants included, those excluded had lower rates of diabetes (30% vs 41%), statin use (60% vs 73%), and had a lower six-minute walk distance at baseline (312 vs 359 m, p < 0.001).
Tertiles of six-minute walk distance at baseline were 21–326 m (Tertile 1), 327–412 m (Tertile 2), and 412–701 m (Tertile 3). Participants with a poorer six-minute walk distance at baseline were older, included more females, had higher prevalences of hypertension, diabetes, angina, heart failure, stroke, and pulmonary disease, and had higher BMI and lower ABI values compared to participants with a better six-minute walk distance at baseline (Table 1). Participants with a lower extremity event during follow-up were not significantly different in age, race, sex, ABI, BMI, or other comorbidities compared to those without an event (Table 2). Over a mean follow-up of 33 months (SD = 16.5), 177 of the 369 participants (48.0%) developed one or more lower extremity events. Among participants with events, 43 (11.7%) were hospitalized for lower extremity revascularization, 125 (33.9%) experienced an ABI decline of 15% or greater, and nine (2.4%) were hospitalized for critical limb ischemia or amputation.
Table 1.
Characteristics of participants with PAD according to baseline six-minute walk distance.
| Total (n = 369) 21–701 m |
Tertile 1 (n = 123) 21–326 m |
Tertile 2 (n = 124) 327–412 m |
Tertile 3 (n = 122) 412–701 m |
Trend p-value |
|
|---|---|---|---|---|---|
| Age, years | 69.4 (10.0) | 71.96 (10.1) | 69.44 (9.9) | 66.70 (9.4) | < 0.0001 |
| Female | 114 (30.9) | 45 (36.6) | 40 (32.3) | 29 (23.8) | 0.0300 |
| African American | 111 (30.1) | 41 (33.3) | 39 (31.5) | 31 (25.4) | 0.18 |
| ABI | 0.67 (0.17) | 0.59 (0.17) | 0.69 (0.17) | 0.74 (0.15) | < 0.0001 |
| BMI, kg/m2 | 29.7 (6.1) | 31.12 (6.8) | 29.61 (6.1) | 28.22 (5.0) | 0.0002 |
| Smoker: current or former | 329 (89.2) | 109 (88.6) | 111 (89.5) | 109 (89.3) | 0.85 |
| Hypertension | 334 (90.5) | 118 (95.9) | 114 (91.9) | 102 (83.6) | 0.001 |
| Diabetes | 150 (40.7) | 65 (52.9) | 49 (39.5) | 36 (29.5) | < 0.001 |
| Angina | 88 (23.8) | 35 (28.5) | 32 (25.8) | 21 (17.2) | 0.039 |
| Myocardial infarction | 74 (20.1) | 27 (22.0) | 24 (19.4) | 23 (18.9) | 0.54 |
| Heart failure | 45 (12.2) | 28 (22.8) | 13 (10.5) | 4 (3.3) | < 0.0001 |
| Stroke | 60 (16.3) | 27 (22.0) | 20 (16.1) | 13 (10.7) | 0.017 |
| Pulmonary disease | 139 (37.7) | 59 (48.0) | 46 (37.1) | 34 (27.9) | 0.001 |
| Cancer | 54 (14.6) | 18 (14.6) | 18 (14.5) | 18 (14.8) | 0.98 |
| LDL, mg/dL | 91.7 (32.3) | 89.70 (33.3) | 91.11 (32.5) | 94.37 (31.2) | 0.26 |
| HDL, mg/dL | 49.4 (16.4) | 47.40 (14.6) | 50.08 (19.9) | 50.85 (14.1) | 0.10 |
| Statin use | 271 (73.4) | 90 (73.2) | 98 (79.0) | 83 (68.0) | 0.36 |
| Six-minute walk distance, m | 359.3 (122.9) | 221.84 (81.7) | 372.41 (22.9) | 484.53 (59.1) | < 0.0001 |
| Reported blocks walked in the past week | 26.8 (56.1) | 9.60 (14.7) | 19.79 (42.9) | 51.48 (80.9) | < 0.0001 |
For continuous variables, data are in the form: mean (SD); for categorical variables: n (%).
ABI, ankle-brachial index; BMI, body mass index; HDL, high density lipoprotein; LDL, low density lipoprotein, PAD, peripheral artery disease.
Table 2.
Baseline characteristics of participants according to whether they experienced a lower extremity atherosclerotic event at follow-up.
| No lower extremity events during follow-up (n = 192) |
With lower extremity events during follow-up (n = 177) |
p-value | |
|---|---|---|---|
| Age, years | 69.52 (9.67) | 69.21 (10.37) | 0.77 |
| Female | 55 (28.65) | 59 (33.33) | 0.33 |
| African American | 60 (31.25) | 51 (28.81) | 0.61 |
| ABI at baseline | 0.66 (0.18) | 0.68 (0.16) | 0.42 |
| BMI at baseline, kg/m2 | 30.18 (6.43) | 29.09 (5.69) | 0.09 |
| Smoker: current or former | 173 (90.10) | 156 (88.14) | 0.54 |
| Hypertension | 170 (88.54) | 164 (92.66) | 0.18 |
| Diabetes | 84 (43.75) | 66 (37.29) | 0.21 |
| Angina | 48 (25.00) | 40 (22.60) | 0.59 |
| Myocardial infarction | 35 (18.23) | 39 (22.03) | 0.36 |
| Heart failure | 22 (11.46) | 23 (12.99) | 0.65 |
| Stroke | 37 (19.27) | 23 (12.99) | 0.10 |
| Pulmonary disease | 77 (40.10) | 62 (35.03) | 0.31 |
| Cancer | 31 (16.15) | 23 (12.99) | 0.39 |
| LDL cholesterol | 91.54 (33.14) | 91.91 (31.46) | 0.91 |
| HDL cholesterol | 48.58 (15.41) | 50.37 (17.49) | 0.30 |
| Log, LDL/HDL | 0.62 (0.42) | 0.60 (0.45) | 0.70 |
| Statin use | 142.00 (73.96) | 129.00 (72.88) | 0.82 |
| Six-minute walk distance, m | 363.60 (124.11) | 354.62 (121.77) | 0.48 |
| Blocks walked | 31.08 (62.67) | 22.19 (47.67) | 0.13 |
For continuous variables, data are in the form: mean (SD); for categorical variables: n (%).
ABI, ankle–brachial index; BMI, body mass index; HDL, high density lipoprotein; LDL, low density lipoprotein.
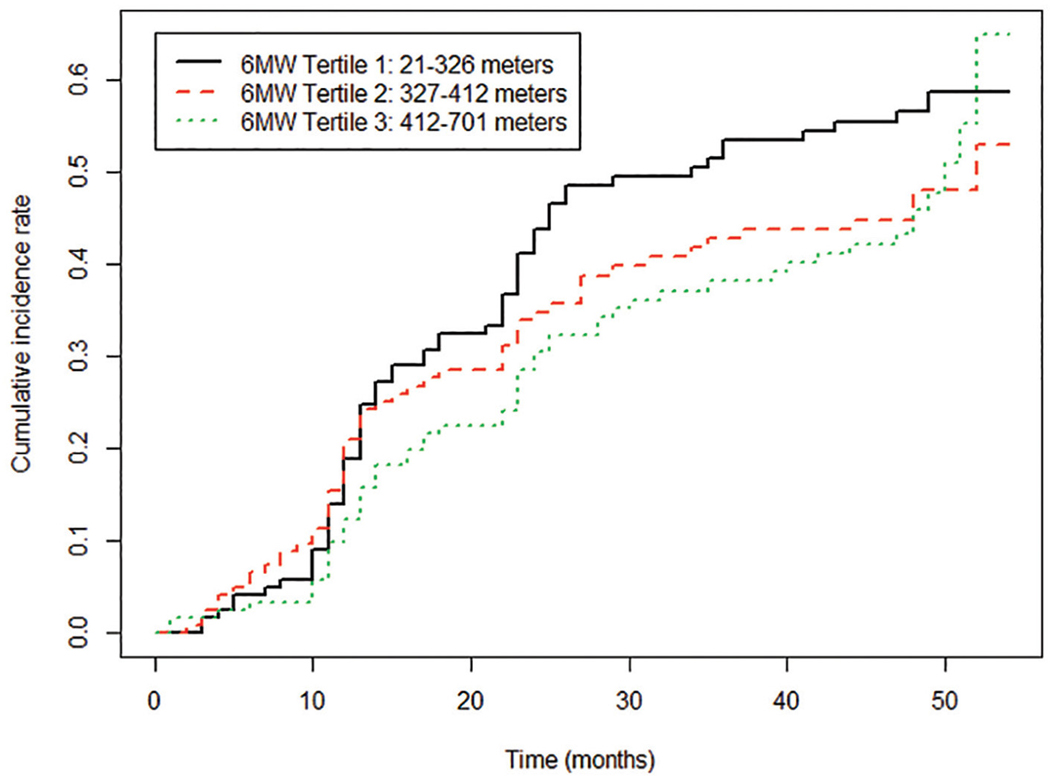
Rates of lower extremity events were 66 of 123 (53.7%) among participants in the first (worst) six-minute walk tertile, 55 of 124 (44.4%) in the second tertile, and 56 of 122 (45.9%) among participants in the third (best) tertile of six-minute walk performance. In unadjusted analyses, there were no significant associations of six-minute walk tertile and lower extremity event rates (Figure 1). However, after adjusting for age, sex, race, BMI, smoking, comorbidities, cholesterol, and statin use, the first (poorest) tertile was associated with a significantly higher rate of lower extremity events compared to the third (best) tertile (hazard ratio (HR) = 1.59, 95% CI 1.09–2.33, p = 0.0166) (Table 3). After additional adjustment for baseline ABI, the association of baseline six-minute walk distance with subsequent lower extremity events remained statistically significant (HR = 1.74, 95% CI 1.17–2.60, p = 0.0067) (Table 3). Analyses remained statistically significant when events due to critical limb ischemia were excluded and when events due to lower extremity revascularization were excluded, respectively (see online supplemental Tables 1 and 2). Analyses remained statistically significant when repeated among participants with ABI < 0.90 (online supplemental Tables 3 and 4).
Figure 1.
Cumulative incidence of combined lower extremity events, for each tertile, with death from any cause as a competing risk factor. 6MW: 6-minute walk.
Table 3.
Association of six-minute walk performance with lower extremity events with and without adjustment for ABI.
| Tertile 1 HR (95% CI) (21–326 m) n = 123 |
p-value | Tertile 2 HR (95% CI) (327–412 m) n = 124 |
p-value | Tertile 3 HR (95% CI) (412–701 m) n = 122 |
|
|---|---|---|---|---|---|
| Model 1 | |||||
| All lower extremity outcomes | 1.59 (1.09, 2.33) n = 66 |
0.0166 | 1.13 (0.77, 1.65) n = 55 |
0.54 | Ref. n = 56 |
| Model 2 | |||||
| All lower extremity outcomes | 1.74 (1.17, 2.60) |
0.0067 | 1.16 (0.79, 1.70) |
0.44 | Ref. |
| Model 1 | |||||
| Lower extremity revascularization | 1.89 (0.96, 3.71) n = 21 |
0.06 | 1.48 (0.77, 2.86) n = 20 |
0.24 | Ref. n = 17 |
| Model 2 | |||||
| Lower extremity revascularization | 1.96 (0.96, 4.01) |
0.06 | 1.50 (0.77, 2.90) |
0.23 | Ref. |
| Model 1 | |||||
| ABI decline > 15%a | 1.51 (0.98, 2.32) n = 52 |
0.06 | 1.01 (0.65, 1.55) n = 41 |
0.97 | Ref. n = 44 |
| Model 2 | |||||
| ABI decline > 15%a | 1.77 (1.12, 2.78) |
0.0136 | 1.08 (0.70, 1.68) |
0.72 | Ref. |
| Model 1 | |||||
| Critical limb ischemia or amputation | 5.52 (0.89, 34.23) n = 7 |
0.07 | 2.53 (0.38, 17.06) n = 4 |
0.34 | Ref. n = 2 |
| Model 2 | |||||
| Critical limb ischemia or amputation | 4.78 (0.70, 32.67) |
0.11 | 2.45 (0.36, 16.69) |
0.36 | Ref. |
Model 1 adjusteding for age, sex, race, BMI, smoking, comorbidities, and statin use. Model 2 adjusted for variables in Model 1 + ABI.
Four people are not included in the analyses for ABI decline > 15%: one in tertile 1, two in tertile 2, and one in tertile 3.
ABI, ankle–brachial index; BMI, body mass index; HR, hazard ratio.
In unadjusted analyses, there were no significant associations of baseline six-minute walk tertile with hospitalization for lower extremity revascularization, ABI decline > 15%, or hospitalization for critical limb ischemia or amputation (online supplemental Figures 1–3). Adjusting for age, sex, race, BMI, smoking, comorbidities, statin use, and ABI, participants in the first (worst) tertile of the six-minute walk test had a significantly higher rate of meaningful ABI decline compared to participants in the third (best) tertile (HR = 1.77, CI 1.12–2.78, p = 0.0136). In adjusted analyses, the six-minute walk distance was not associated significantly with lower extremity revascularization or hospitalization for critical limb ischemia (Table 3).
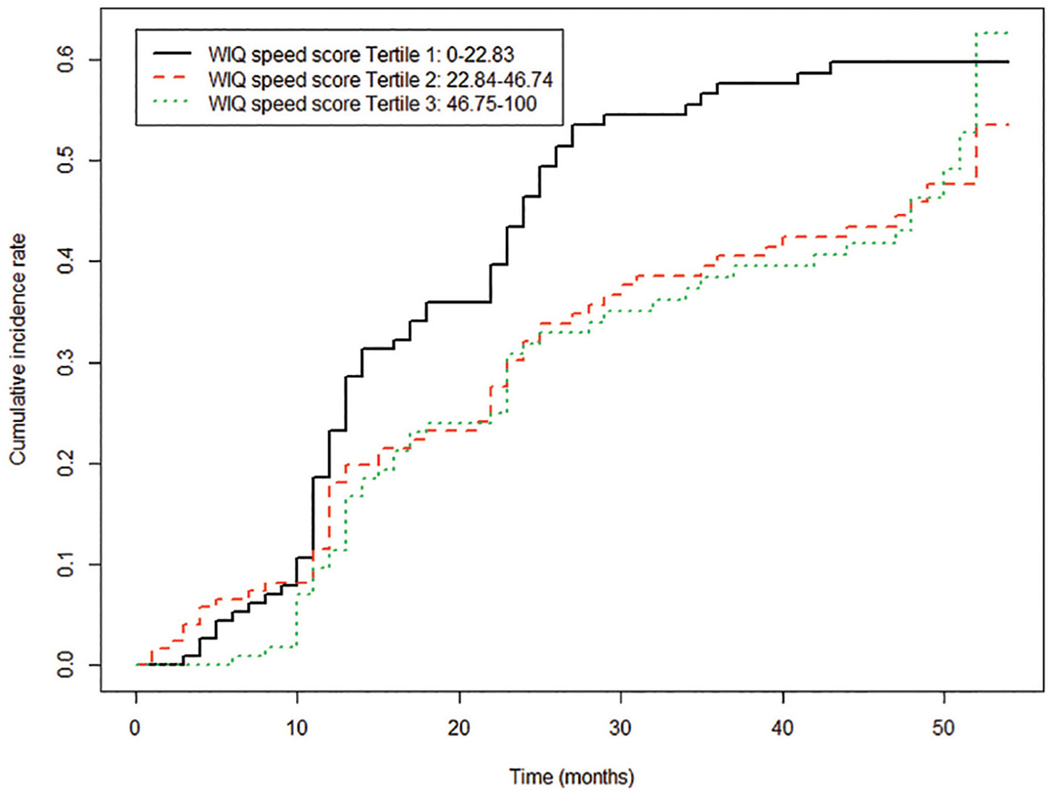
In unadjusted analyses, there were no significant associations of baseline WIQ speed score tertiles with lower extremity event rates (Figure 2). However, after adjusting for age, sex, race, BMI, smoking, comorbidities, and statin use (HR = 1.62, 95% CI 1.11–2.37, p = 0.0122), the first (worst) tertile of walking speed score was associated significantly with higher rates of lower extremity events compared to the third (best) tertile. Results remained statistically significant after additional adjustment for ABI (HR = 1.80, 95% CI 1.20–2.69, p = 0.0041) (Table 4). In addition, participants in the first (worst) tertile of walking speed score had significantly higher rates of meaningful ABI decline compared to participants in the third (best) tertile, after adjusting for age, sex, race, BMI, smoking, comorbidities, and statin use (HR = 1.56, 95% CI 1.02–2.39, p = 0.0410). Results remained statistically significant after additional adjustment for ABI (HR = 1.88, 95% CI 1.19–2.98, p = 0.0070). In adjusted analyses, those in the first (worst) tertile of walking speed score also had significantly higher rates of hospitalization for lower extremity revascularization compared to the third (best) tertile (Table 4). The WIQ walking speed score tertile was not significantly associated with hospitalization for the combined outcome of critical limb ischemia or amputation (Table 4). There were no significant associations of baseline WIQ distance score, WIQ stair climbing score, or physical activity with lower extremity event rates (see online supplemental tables 5–7). There were no statistically significant associations of the time or distance to claudication onset, number of rests, or total rest time during the six-minute walk test with lower extremity events (online supplemental Table 8). In post hoc analyses, HRs for the association of lowest versus highest six-minute walk tertile with combined lower extremity outcomes were similar between African Americans (n = 111) (HR = 1.634, 95% CI 0.457–5.84, p = 0.450) and non-African Americans (n = 258) (HR = 1.764, 95% CI 0.75–4.16, p = 0.194).
Figure 2.
Cumulative incidence of combined lower extremity events for each tertile of baseline Walking Impairment Questionnaire (WIQ) speed score, with death from any cause as a competing risk factor.
Table 4.
Association of WIQ speed score with lower extremity events, with and without adjustment for ABI.
| Tertile 1 HR (95% CI) (0.0–22.8) n = 113 |
Pairwise p-value relative to 3rd tertile | Tertile 2 HR (95% CI) (25.0–46.7) n = 122 |
Pairwise p-value relative to 3rd tertile | Tertile 3 HR (95% CI) (50.0–100) n = 114 |
|
|---|---|---|---|---|---|
| Model 1 | |||||
| All lower extremity outcomes | 1.62 (1.11, 2.37) n = 64 |
0.0122 | 0.94 (0.63, 1.39) n = 54 |
0.75 | Ref. n = 51 |
| Model 2 | |||||
| All lower extremity outcomes | 1.80 (1.20, 2.69) |
0.0041 | 0.98 (0.66, 1.46) |
0.93 | Ref. |
| Model 1 | |||||
| Lower extremity revascularization | 2.17 (1.05, 4.49) n = 21 |
0.0366 | 2.00 (0.98, 4.05) n = 23 |
0.06 | Ref. n = 12 |
| Model 2 | |||||
| Lower extremity revascularization | 2.22 (1.04, 4.71) |
0.0390 | 2.01 (0.99, 4.10) |
0.055 | Ref. |
| Model 1 | |||||
| ABI decline > 15%a | 1.56 (1.02, 2.39) n = 51 |
0.0410 | 0.82 (0.52, 1.29) n = 39 |
0.39 | Ref. n = 40 |
| Model 2 | |||||
| ABI decline > 15%a | 1.88 (1.19, 2.98) |
0.0070 | 0.90 (0.57, 1.42) |
0.64 | Ref. |
| Model 1 | |||||
| Critical limb ischemia or amputation | 3.48 (0.69, 17.51) n = 7 |
0.13 | 1.54 (0.24, 9.68) n = 3 |
0.65 | Ref. n = 2 |
| Model 2 | |||||
| Critical limb ischemia or amputation | 3.10 (0.58, 16.64) |
0.19 | 1.48 (0.23, 9.39) |
0.68 | Ref. |
Model 1 adjusteding for age, sex, race, BMI, smoking, comorbidities, and statin use. Model 2 adjusted for variables in Model 1 + ABI.
Three people are not included in the analyses for ABI decline > 15%: two in tertile 2, and one in tertile 3.
ABI, ankle–brachial index; BMI, body mass index; HR, hazard ratio; WIQ, Walking Impairment Questionnaire.
Discussion
Among 369 people with PAD, 177 (48.0%) developed one or more lower extremity events during a mean follow-up of 33 months. Poorer baseline six-minute walk distance was associated with a higher incidence of lower extremity events after adjusting for potential confounders, including baseline ABI. Participants in the lowest six-minute walk distance tertile had a 1.74-fold higher risk of all lower extremity events compared with those in the best tertile at baseline, adjusting for ABI and other confounders. The WIQ distance score, stair climbing score, physical activity, and characteristics of the six-minute walk test, such as total number of rests or time or distance to onset of ischemic leg symptoms, were not associated with subsequent lower extremity events. Lower WIQ speed scores at baseline were associated with a significantly higher rate of lower extremity events compared to higher WIQ speed scores at baseline. An additional important finding was the high rate of lower extremity events in people with PAD.
In 2007, McDermott et al. reported that PAD participants in the lowest six-minute walk distance quartile had a nearly 10-fold higher rate of mobility loss and a six-fold higher rate of becoming unable to walk for 6 minutes continuously, compared to those in the best two quartiles for six-minute walk distance at baseline.3 In 2008, McDermott et al. reported that among people with PAD, a poorer six-minute walk distance was associated with significantly higher rates of all-cause mortality and cardiovascular disease mortality. In 2015, McDermott et al. reported that among people with PAD, a poorer six-minute walk distance was associated with higher rates of ischemic heart disease events and stroke.2,4 To our knowledge, the association of six-minute walk distance with subsequent lower extremity events has not been reported previously. This study included a large proportion of African Americans. Results did not substantially differ by presence versus absence of African American race.
In the fully adjusted models, compared to the best six-minute walk distance tertile at baseline, the point estimate for the association of the lowest tertile of six-minute walk distance at baseline with the combined outcome of critical limb ischemia or amputation was 4.78, while the point estimate for the association of six-minute walk distance with a 15% decline in the ABI was 1.77. This difference in the strength of associations between baseline six-minute walk distance and critical limb ischemia or amputation versus a 15% decline in the ABI may be due to the fact that participants with a better six-minute walk distance at baseline had higher ABI values at baseline, and prior study showed that higher ABI values were more likely to decline during follow-up, perhaps due to a floor effect for people with lower ABI values at baseline.23,25 Statistical adjustment may not have completely overcome this confounding, potentially weakening the observed association of six-minute walk distance with lower extremity events. The absence of a statistically significant association of baseline six-minute walk distance with the development of critical limb ischemia or amputation may have been due to the small number of these lower extremity events, which limited statistical power and increased the confidence interval. However, it is also possible that even with lower events no association of baseline six-minute walk distance with development of critical limb ischemia or amputation would be observed.
The six-minute walk distance is simple to measure and requires only a stopwatch and a 30.48-meter hallway. Previous research demonstrated that the six-minute walk significantly improves in response to supervised treadmill exercise and home-based walking exercise in individuals with PAD.3–5,8,26,27 Future research is needed to determine whether interventions that improve six-minute walk distance can prevent lower extremity events in people with PAD.
Limitations
This study has limitations. First, data are observational, and no causal inferences can be made. Second, the observational nature of the study may have resulted in unmeasured confounding. Third, participants in the lowest tertile of six-minute walk distance at baseline were older, included a higher proportion of people with diabetes, and had lower ABI values than those in the highest tertile at baseline. Residual confounding may contribute to our findings. Fourth, lower extremity revascularization is a relatively subjective clinical outcome because variability exists in clinician judgement regarding decisions to perform lower extremity revascularization.28 However, six-minute walk distance remained significantly associated with lower extremity events when lower extremity revascularization was excluded from the analysis. In addition, most of the lower extremity events were due to ABI decline. Fifth, there were few critical limb ischemia or amputation outcomes, limiting statistical power to identify significant associations for these outcomes. Sixth, the significant associations of a poorer six-minute walk distance with higher rates of LE events were primarily influenced by the relatively high incidence of lower extremity revascularizations and decline in ABI value. These outcomes are less severe than critical limb ischemia or amputation. Seventh, of the original WALCS cohort, 104 (22%) could not be included in these analyses because of death or drop-out before 1-year follow-up or because of missing covariate data. Results may not be generalizable to those not included. Eighth, the study took place in an urban setting; results may not be generalizable to non-urban settings.
Conclusion
A large proportion of people with PAD develop a lower extremity event over time. Poorer baseline six-minute walk distance is associated with higher rates of lower extremity events, after adjusting for the ABI and other potential confounders. Further study is needed to determine whether interventions that improve six-minute walk distance, such as exercise, can prevent the progression of lower extremity events in people with PAD.
Supplementary Material
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was funded by the National Heart, Lung, and Blood Institute (R01-HL083064 and R01-109244) and by intramural support from the National Institute on Aging and the Jesse Brown VA Medical Center.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr McDermott has received research support from Regeneron, the Hershey Company, Chromadex, Reserveage, Mars, and Helixmith.
Footnotes
Supplementary material
The supplementary material is available online with the article.
Declaration of conflicting interests
The other authors have no disclosures.
References
- 1.Gardner AW, Montgomery PS, Wang M. Minimal clinically important differences in treadmill, 6-minute walk, and patient-based outcomes following supervised and home-based exercise in peripheral artery disease. Vasc Med 2018; 23: 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDermott MM, Greenland P, Tian L, et al. Association of 6-minute walk performance and physical activity with incident ischemic heart disease events and stroke in peripheral artery disease. J Am Heart Assoc 2015; 4: e001846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDermott MM, Guralnik JM, Tian L, et al. Baseline functional performance predicts the rate of mobility loss in persons with peripheral arterial disease. J Am Coll Cardiol 2007; 50: 974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott MM, Tian L, Liu K, et al. Prognostic value of functional performance for mortality in patients with peripheral artery disease. J Am Coll Cardiol 2008; 51: 1482–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins TC, Lu L, Ahluwalia JS, et al. Efficacy of community-based exercise therapy among African American patients with peripheral artery disease: A randomized clinical trial. JAMA Netw Open 2019; 2: e187959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulinska K, Kropielnicka K, Jasinski T, et al. Nordic pole walking improves walking capacity in patients with intermittent claudication: A randomized controlled trial. Disabil Rehabil 2016; 38: 1318–1324. [DOI] [PubMed] [Google Scholar]
- 7.Gardner AW, Parker DE, Montgomery PS, et al. Step-monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: A randomized controlled trial. J Am Heart Assoc 2014; 3: e001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordanstig J, Broeren M, Hensater M, et al. Six-minute walk test closely correlates to ‘real-life’ outdoor walking capacity and quality of life in patients with intermittent claudication. J Vasc Surg 2014; 60: 404–409. [DOI] [PubMed] [Google Scholar]
- 9.McDermott MM, Liu K, Carr J, et al. Superficial femoral artery plaque, the ankle–brachial index, and leg symptoms in peripheral arterial disease: The Walking and Leg Circulation Study (WALCS) III. Circ Cardiovasc Imaging 2011; 4: 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017; 135: e686–e725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heun R, Papassotiropoulos A, Jennssen F. The validity of psychometric instruments for detection of dementia in the elderly general population. Int J Geriatr Psychiatry 1998; 13: 368–380. [DOI] [PubMed] [Google Scholar]
- 12.Aboyans V, Criqui MH, Abraham P, et al. ; American Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention; and Council on Cardiovascular Surgery and Anesthesia. Measurement and interpretation of the ankle–brachial index: A scientific statement from the American Heart Association. Circulation 2012; 126: 2890–2909. [DOI] [PubMed] [Google Scholar]
- 13.McDermott MM, Criqui MH, Liu K, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg 2000; 32: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 14.Guralnik JM, Fried LP, Simonsick EM, et al. The Women’s Health and Aging Study: Health and social characteristics of older women with disability. Bethesda, MD: National Institute on Aging; 1995. NIH publication No. 95–4009, Appendix E. [Google Scholar]
- 15.Regensteiner JG, Steiner JF, Panzer RJ, et al. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol 1990; 2: 142–152. [Google Scholar]
- 16.McDermott MM, Ohlmiller SM, Liu K, et al. Gait alterations associated with walking impairment in people with peripheral arterial disease with and without intermittent claudication. J Am Geriatr Soc 2001; 49: 747–754. [DOI] [PubMed] [Google Scholar]
- 17.Garg P, Tian L, Criqui M. Physical activity during daily life and mortality in patients with peripheral arterial disease. Circulation 2006;114:242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson M, Leon A, Jacobs D, et al. Ability of the Caltrac accelerometer to assess daily physical activity levels. J Cardiopulm Rehabil 1995; 15: 107–113. [DOI] [PubMed] [Google Scholar]
- 19.Miller DJ, Freedson PS, Kline GM. Comparison of activity levels using the Caltrac accelerometer and five questionnaires. Med Sci Sports Exerc 1994; 26: 376–382. [PubMed] [Google Scholar]
- 20.Sallis JF, Buono MJ, Roby JJ, et al. The Caltrac accelerometer as a physical activity monitor for school aged children. Med Sci Sports Exerc 1990; 22: 698–703. [DOI] [PubMed] [Google Scholar]
- 21.Rifai N, Iannotti E, DeAnagelis K, et al. Analytical and clinical performance of a homogenous enzymatic LDL-cholesterol assay compared with the ultracentrifugation-dextran sulfate-Mg2+ method. Clin Chem 1998; 44: 1242–1250. [PubMed] [Google Scholar]
- 22.Sugiuchi H, Ugi Y, Okabe H, et al. Direct measurement of high-density lipoprotein cholesterol in serum with polyethylene glycol-modified enzymes and sulfated a-cyclodextrin. Clin Chem 1995; 41: 717–723. [PubMed] [Google Scholar]
- 23.Forbang NI, Mcdermott MM, Liao Y, et al. Associations of diabetes mellitus and other cardiovascular disease risk factors with decline in the ankle–brachial index. Vasc Med 2014; 19: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLafferty RB, Moneta GL, Taylor LM, et al. Ability of ankle–brachial index to detect lower-extremity atherosclerotic disease progression. Arch Surg 1997; 132: 836–841. [DOI] [PubMed] [Google Scholar]
- 25.McDermott MM, Kramer CM, Tian L, et al. Plaque composition in the proximal superficial femoral artery and peripheral artery disease events. J Am Coll Cardiol Imaging 2017; 10: 1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: A randomized controlled trial. JAMA 2009; 301: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease: A randomized clinical trial. JAMA 2013; 310: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soden PA, Zettervall SL, Curran T, et al. Regional variation in patient selection and treatment for lower extremity vascular disease in the Vascular Quality Initiative. J Vasc Surg 2017; 65: 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.