Abstract
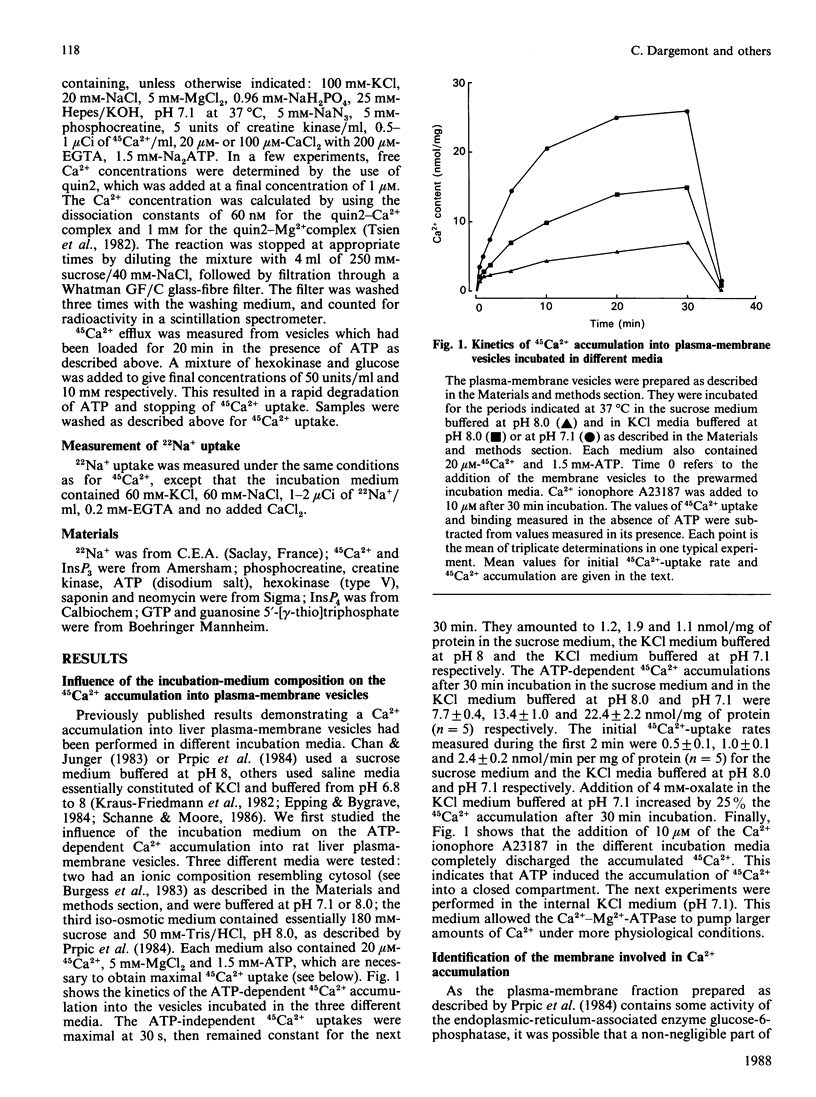
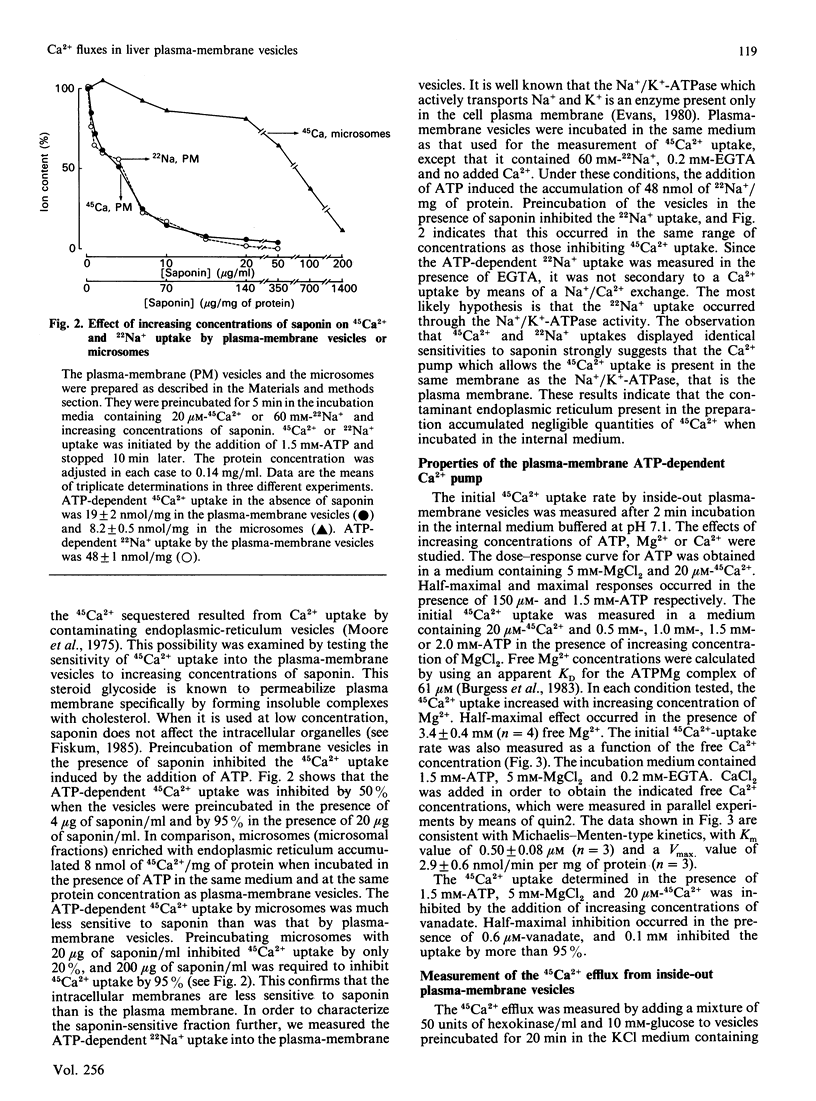
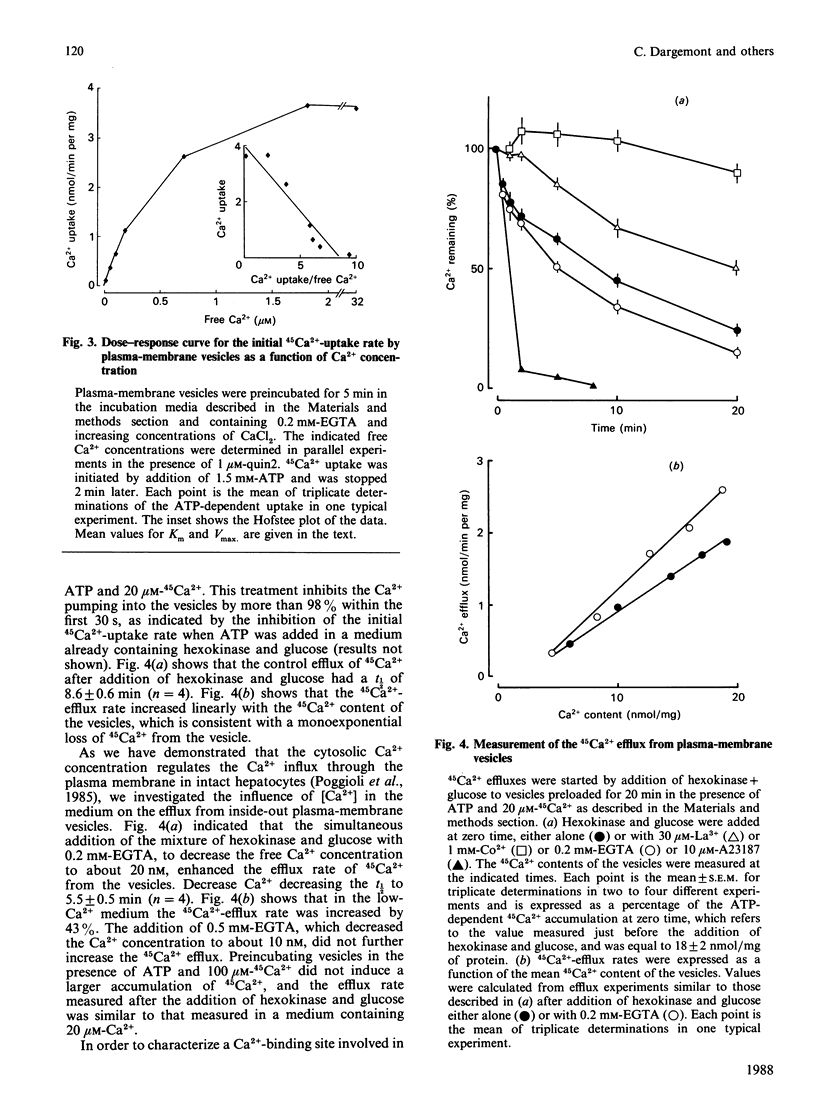
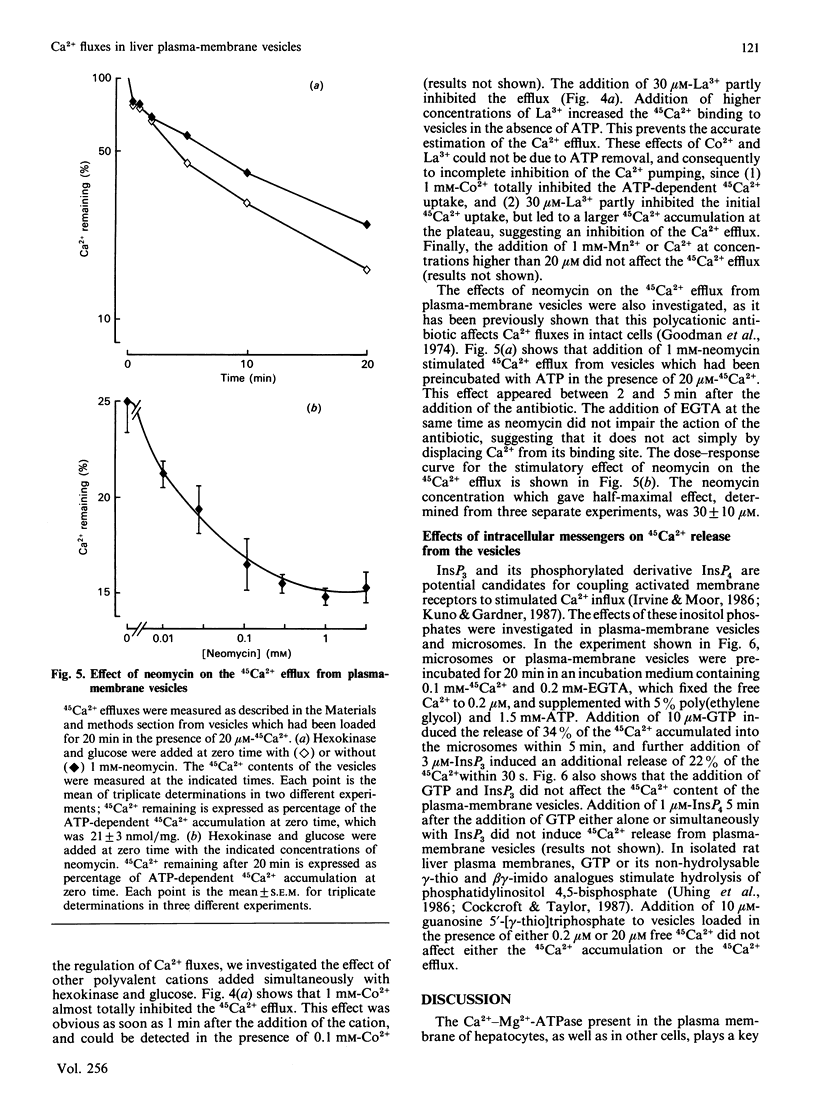
Inside-out plasma-membrane vesicles isolated from rat liver [Prpic, Green, Blackmore & Exton (1984) J. Biol. Chem. 259, 1382-1385] accumulated a substantial amount of 45Ca2+ when they were incubated in a medium whose ionic composition and pH mimicked those of cytosol and which contained MgATP. The Vmax of the initial 45Ca2+ uptake rate was 2.9 +/- 0.6 nmol/min per mg and the Km for Ca2+ was 0.50 +/- 0.08 microM. The ATP-dependent 45Ca2+ uptake by inside-out plasma-membrane vesicles was about 20 times more sensitive to saponin than was the ATP-dependent uptake by a microsomal preparation. The 45Ca2+ efflux from the inside-out vesicles, which is equivalent to the Ca2+ influx in intact cells, was increased when the free Ca2+ concentration in the medium was decreased. The Ca2+ antagonists La3+ and Co2+ inhibited the 45Ca2+ efflux from the vesicles. Neomycin stimulated the Ca2+ efflux in the presence of either a high or a low free Ca2+ concentration. These results confirm that polyvalent cations regulate Ca2+ fluxes through the plasma membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altin J. G., Bygrave F. L. The influx of Ca2+ induced by the administration of glucagon and Ca2+-mobilizing agents to the perfused rat liver could involve at least two separate pathways. Biochem J. 1987 Feb 15;242(1):43–50. doi: 10.1042/bj2420043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Berthon B., Binet A., Mauger J. P., Claret M. Cytosolic free Ca2+ in isolated rat hepatocytes as measured by quin2. Effects of noradrenaline and vasopressin. FEBS Lett. 1984 Feb 13;167(1):19–24. doi: 10.1016/0014-5793(84)80824-8. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Fabiato A., Leslie B. A., Putney J. W., Jr Calcium pools in saponin-permeabilized guinea pig hepatocytes. J Biol Chem. 1983 Dec 25;258(24):15336–15345. [PubMed] [Google Scholar]
- Chan K. M., Junger K. D. Calcium transport and phosphorylated intermediate of (Ca2+ + Mg2+)-ATPase in plasma membranes of rat liver. J Biol Chem. 1983 Apr 10;258(7):4404–4410. [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Berthon B., Exton J. H. Changes in free cytosolic Ca2+ in hepatocytes following alpha 1-adrenergic stimulation. Studies on Quin-2-loaded hepatocytes. J Biol Chem. 1983 Jul 25;258(14):8769–8773. [PubMed] [Google Scholar]
- Cockcroft S., Taylor J. A. Fluoroaluminates mimic guanosine 5'-[gamma-thio]triphosphate in activating the polyphosphoinositide phosphodiesterase of hepatocyte membranes. Role for the guanine nucleotide regulatory protein Gp in signal transduction. Biochem J. 1987 Jan 15;241(2):409–414. doi: 10.1042/bj2410409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combettes L., Berthon B., Binet A., Claret M. Glucagon and vasopressin interactions on Ca2+ movements in isolated hepatocytes. Biochem J. 1986 Aug 1;237(3):675–683. doi: 10.1042/bj2370675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Comerford J. G., Fulton D. V. The effect of GTP on inositol 1,4,5-trisphosphate-stimulated Ca2+ efflux from a rat liver microsomal fraction. Is a GTP-dependent protein phosphorylation involved? Biochem J. 1986 Mar 1;234(2):311–315. doi: 10.1042/bj2340311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Irvine R. F. Inositol (1,4,5)trisphosphate-promoted Ca2+ release from microsomal fractions of rat liver. Biochem Biophys Res Commun. 1984 May 16;120(3):858–864. doi: 10.1016/s0006-291x(84)80186-2. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping R. J., Bygrave F. L. A procedure for the rapid isolation from rat liver of plasma membrane vesicles exhibiting Ca2+-transport and Ca2+-ATPase activities. Biochem J. 1984 Nov 1;223(3):733–745. doi: 10.1042/bj2230733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H. A biochemical dissection of the functional polarity of the plasma membrane of the hepatocyte. Biochim Biophys Acta. 1980 May 27;604(1):27–64. doi: 10.1016/0005-2736(80)90584-2. [DOI] [PubMed] [Google Scholar]
- Fiskum G. Intracellular levels and distribution of Ca2+ in digitonin-permeabilized cells. Cell Calcium. 1985 Apr;6(1-2):25–37. doi: 10.1016/0143-4160(85)90032-6. [DOI] [PubMed] [Google Scholar]
- Goodman F. R., Weiss G. B., Adams H. R. Alterations by neomycin of 45Ca movements and contractile responses in vascular smooth muscle. J Pharmacol Exp Ther. 1974 Feb;188(2):472–480. [PubMed] [Google Scholar]
- Guillemette G., Balla T., Baukal A. J., Catt K. J. Characterization of inositol 1,4,5-trisphosphate receptors and calcium mobilization in a hepatic plasma membrane fraction. J Biol Chem. 1988 Apr 5;263(10):4541–4548. [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus-Friedmann N., Biber J., Murer H., Carafoli E. Calcium uptake in isolated hepatic plasma-membrane vesicles. Eur J Biochem. 1982 Dec;129(1):7–12. doi: 10.1111/j.1432-1033.1982.tb07014.x. [DOI] [PubMed] [Google Scholar]
- Kuno M., Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987 Mar 19;326(6110):301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- Lipsky J. J., Lietman P. S. Aminoglycoside inhibition of a renal phosphatidylinositol phospholipase C. J Pharmacol Exp Ther. 1982 Feb;220(2):287–292. [PubMed] [Google Scholar]
- Lotersztajn S., Hanoune J., Pecker F. A high affinity calcium-stimulated magnesium-dependent ATPase in rat liver plasma membranes. Dependence of an endogenous protein activator distinct from calmodulin. J Biol Chem. 1981 Nov 10;256(21):11209–11215. [PubMed] [Google Scholar]
- Marche P., Koutouzov S., Girard A. Impairment of membrane phosphoinositide metabolism by aminoglycoside antibiotics: streptomycin, amikacin, kanamycin, dibekacin, gentamicin and neomycin. J Pharmacol Exp Ther. 1983 Nov;227(2):415–420. [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Claret M. Synergistic stimulation of the Ca2+ influx in rat hepatocytes by glucagon and the Ca2+-linked hormones vasopressin and angiotensin II. J Biol Chem. 1985 Sep 25;260(21):11635–11642. [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Guesdon F., Claret M. Noradrenaline, vasopressin and angiotensin increase Ca2+ influx by opening a common pool of Ca2+ channels in isolated rat liver cells. Biochem J. 1984 Jul 1;221(1):121–127. doi: 10.1042/bj2210121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L., Chen T., Knapp H. R., Jr, Landon E. J. Energy-dependent calcium sequestration activity in rat liver microsomes. J Biol Chem. 1975 Jun 25;250(12):4562–4568. [PubMed] [Google Scholar]
- Parker J. C., Barritt G. J. Evidence that lanthanum ions stimulate calcium inflow to isolated hepatocytes. Biochem J. 1981 Oct 15;200(1):109–114. doi: 10.1042/bj2000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggioli J., Mauger J. P., Claret M. Effect of cyclic AMP-dependent hormones and Ca2+-mobilizing hormones on the Ca2+ influx and polyphosphoinositide metabolism in isolated rat hepatocytes. Biochem J. 1986 May 1;235(3):663–669. doi: 10.1042/bj2350663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggioli J., Mauger J. P., Guesdon F., Claret M. A regulatory calcium-binding site for calcium channel in isolated rat hepatocytes. J Biol Chem. 1985 Mar 25;260(6):3289–3294. [PubMed] [Google Scholar]
- Prpić V., Green K. C., Blackmore P. F., Exton J. H. Vasopressin-, angiotensin II-, and alpha 1-adrenergic-induced inhibition of Ca2+ transport by rat liver plasma membrane vesicles. J Biol Chem. 1984 Feb 10;259(3):1382–1385. [PubMed] [Google Scholar]
- Sastrasinh M., Knauss T. C., Weinberg J. M., Humes H. D. Identification of the aminoglycoside binding site in rat renal brush border membranes. J Pharmacol Exp Ther. 1982 Aug;222(2):350–358. [PubMed] [Google Scholar]
- Schanne F. A., Moore L. Liver plasma membrane calcium transport. Evidence for a Na+-dependent Ca2+ flux. J Biol Chem. 1986 Jul 25;261(21):9886–9889. [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhing R. J., Prpic V., Jiang H., Exton J. H. Hormone-stimulated polyphosphoinositide breakdown in rat liver plasma membranes. Roles of guanine nucleotides and calcium. J Biol Chem. 1986 Feb 15;261(5):2140–2146. [PubMed] [Google Scholar]
- Whipps D. E., Armston A. E., Pryor H. J., Halestrap A. P. Effects of glucagon and Ca2+ on the metabolism of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in isolated rat hepatocytes and plasma membranes. Biochem J. 1987 Feb 1;241(3):835–845. doi: 10.1042/bj2410835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Tscharner V., Prod'hom B., Baggiolini M., Reuter H. Ion channels in human neutrophils activated by a rise in free cytosolic calcium concentration. 1986 Nov 27-Dec 3Nature. 324(6095):369–372. doi: 10.1038/324369a0. [DOI] [PubMed] [Google Scholar]


