Abstract
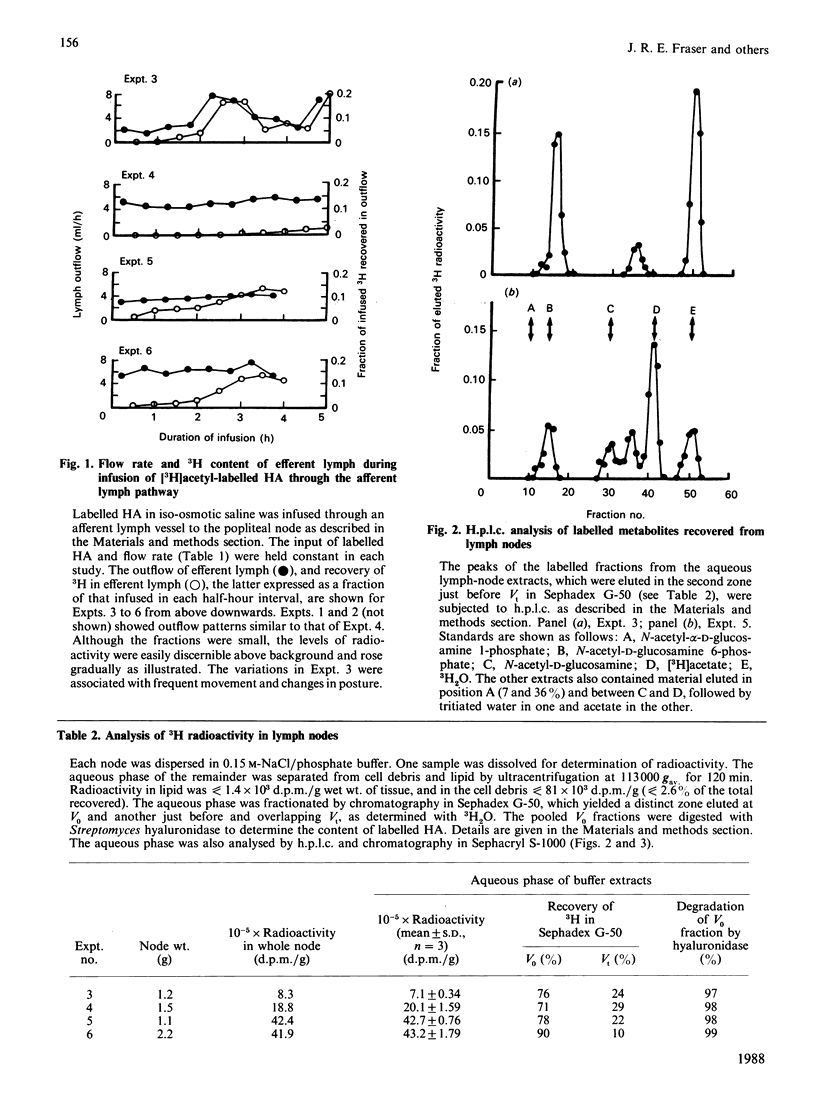
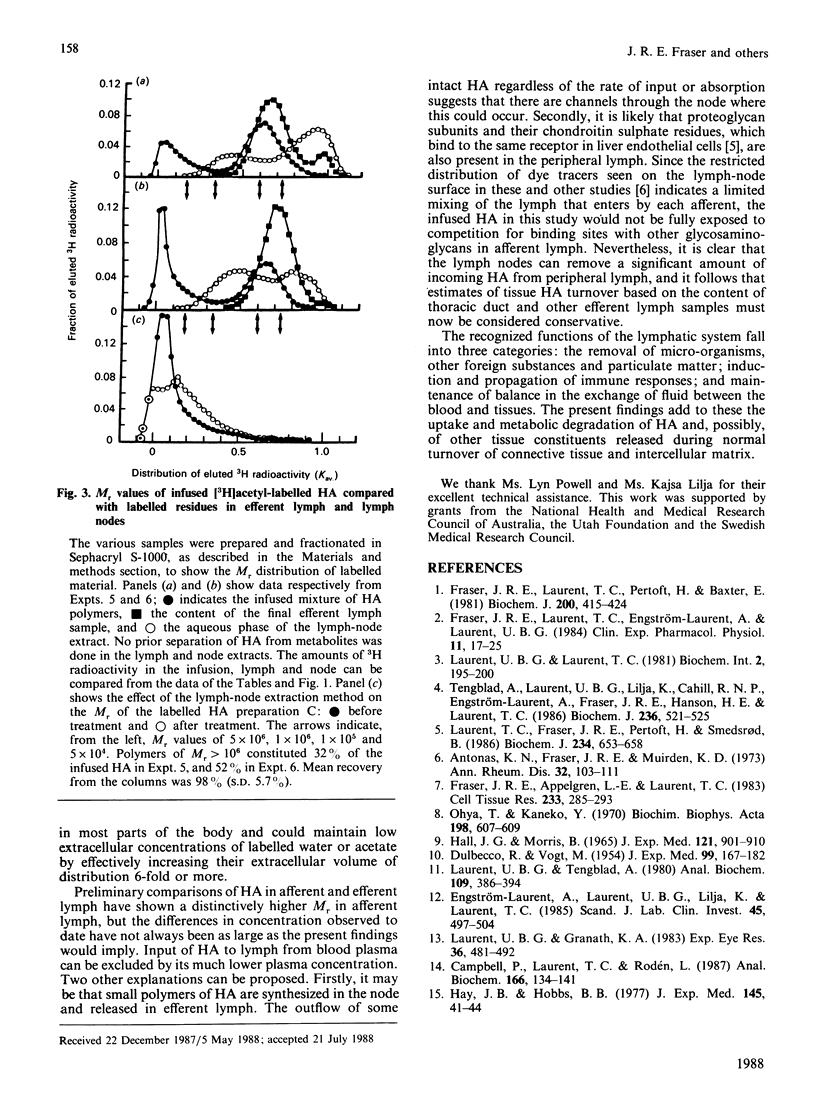
Afferent lymph vessels entering popliteal lymph nodes of sheep were infused with [3H]acetyl-labelled hyaluronan of high Mr (4.3 x 10(6)-5.5 x 10(6)) and low Mr (1.5 x 10(5)). Analysis of efferent lymph and of residues in the nodes showed that hyaluronan presented by this route is taken up and degraded by lymphatic tissue. Labelled residues isolated in node extracts by gel chromatography and h.p.l.c. included N-acetylglucosamine, acetate, water and a fraction provisionally identified as N-acetylglucosamine 6-phosphate. Between 48 and 75% of the infused material was unrecovered, and had been presumably eliminated through the bloodstream as diffusible residues. Rates of degradation reached as high as 43 micrograms/h in a node of 2 g wt. infused with 56 micrograms/h. Some HA passed into efferent lymph and some was detected in the nodes, but fractions of Mr greater than 1 x 10(6) were not found in either. It is concluded that the amounts and Mr values of hyaluronan released from the tissues into peripheral lymph can be significantly underestimated by analysis of efferent lymph, i.e. lymph that has passed through lymph nodes. A substantial role in the normal metabolic turnover of at least one major constituent of intercellular matrix and connective tissue may now be added to the established functions of the lymphatic system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonas K. N., Fraser J. R., Muirden K. D. Distribution of biologically labelled radioactive hyaluronic acid injected into joints. Ann Rheum Dis. 1973 Mar;32(2):103–111. doi: 10.1136/ard.32.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P., Laurent T. C., Rodén L. Assay and properties of N-acetylglucosamine-6-phosphate deacetylase from rat liver. Anal Biochem. 1987 Oct;166(1):134–141. doi: 10.1016/0003-2697(87)90555-0. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström-Laurent A., Laurent U. B., Lilja K., Laurent T. C. Concentration of sodium hyaluronate in serum. Scand J Clin Lab Invest. 1985 Oct;45(6):497–504. doi: 10.3109/00365518509155249. [DOI] [PubMed] [Google Scholar]
- Fraser J. R., Appelgren L. E., Laurent T. C. Tissue uptake of circulating hyaluronic acid. A whole body autoradiographic study. Cell Tissue Res. 1983;233(2):285–293. doi: 10.1007/BF00238296. [DOI] [PubMed] [Google Scholar]
- Fraser J. R., Laurent T. C., Engström-Laurent A., Laurent U. G. Elimination of hyaluronic acid from the blood stream in the human. Clin Exp Pharmacol Physiol. 1984 Jan-Feb;11(1):17–25. doi: 10.1111/j.1440-1681.1984.tb00235.x. [DOI] [PubMed] [Google Scholar]
- Fraser J. R., Laurent T. C., Pertoft H., Baxter E. Plasma clearance, tissue distribution and metabolism of hyaluronic acid injected intravenously in the rabbit. Biochem J. 1981 Nov 15;200(2):415–424. doi: 10.1042/bj2000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL J. G., MORRIS B. THE ORIGIN OF THE CELLS IN THE EFFERENT LYMPH FROM A SINGLE LYMPH NODE. J Exp Med. 1965 Jun 1;121:901–910. doi: 10.1084/jem.121.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay J. B., Hobbs B. B. The flow of blood to lymph nodes and its relation to lymphocyte traffic and the immune response. J Exp Med. 1977 Jan 1;145(1):31–44. doi: 10.1084/jem.145.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent T. C., Fraser J. R., Pertoft H., Smedsrød B. Binding of hyaluronate and chondroitin sulphate to liver endothelial cells. Biochem J. 1986 Mar 15;234(3):653–658. doi: 10.1042/bj2340653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent U. B., Granath K. A. The molecular weight of hyaluronate in the aqueous humour and vitreous body of rabbit and cattle eyes. Exp Eye Res. 1983 Apr;36(4):481–492. doi: 10.1016/0014-4835(83)90042-8. [DOI] [PubMed] [Google Scholar]
- Laurent U. B., Tengblad A. Determination of hyaluronate in biological samples by a specific radioassay technique. Anal Biochem. 1980 Dec;109(2):386–394. doi: 10.1016/0003-2697(80)90665-x. [DOI] [PubMed] [Google Scholar]
- Ohya T., Kaneko Y. Novel hyaluronidase from streptomyces. Biochim Biophys Acta. 1970 Mar 18;198(3):607–609. doi: 10.1016/0005-2744(70)90139-7. [DOI] [PubMed] [Google Scholar]
- Tengblad A., Laurent U. B., Lilja K., Cahill R. N., Engström-Laurent A., Fraser J. R., Hansson H. E., Laurent T. C. Concentration and relative molecular mass of hyaluronate in lymph and blood. Biochem J. 1986 Jun 1;236(2):521–525. doi: 10.1042/bj2360521. [DOI] [PMC free article] [PubMed] [Google Scholar]


