Abstract
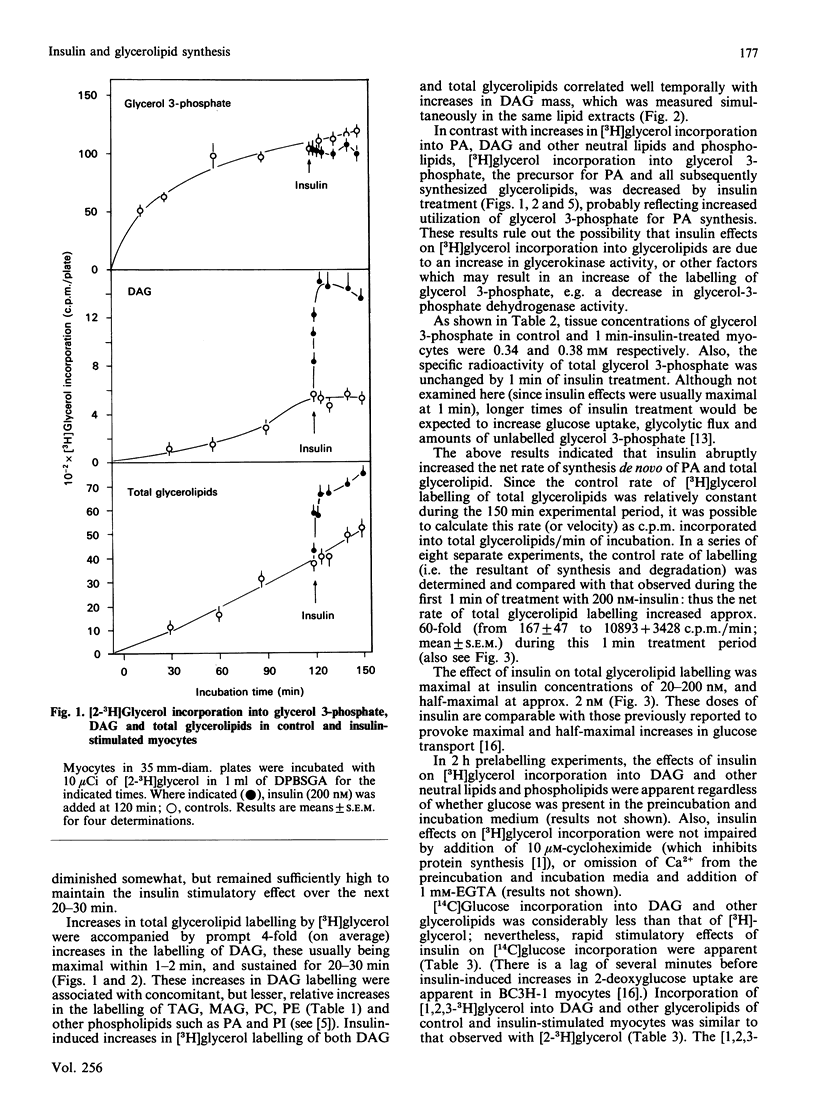
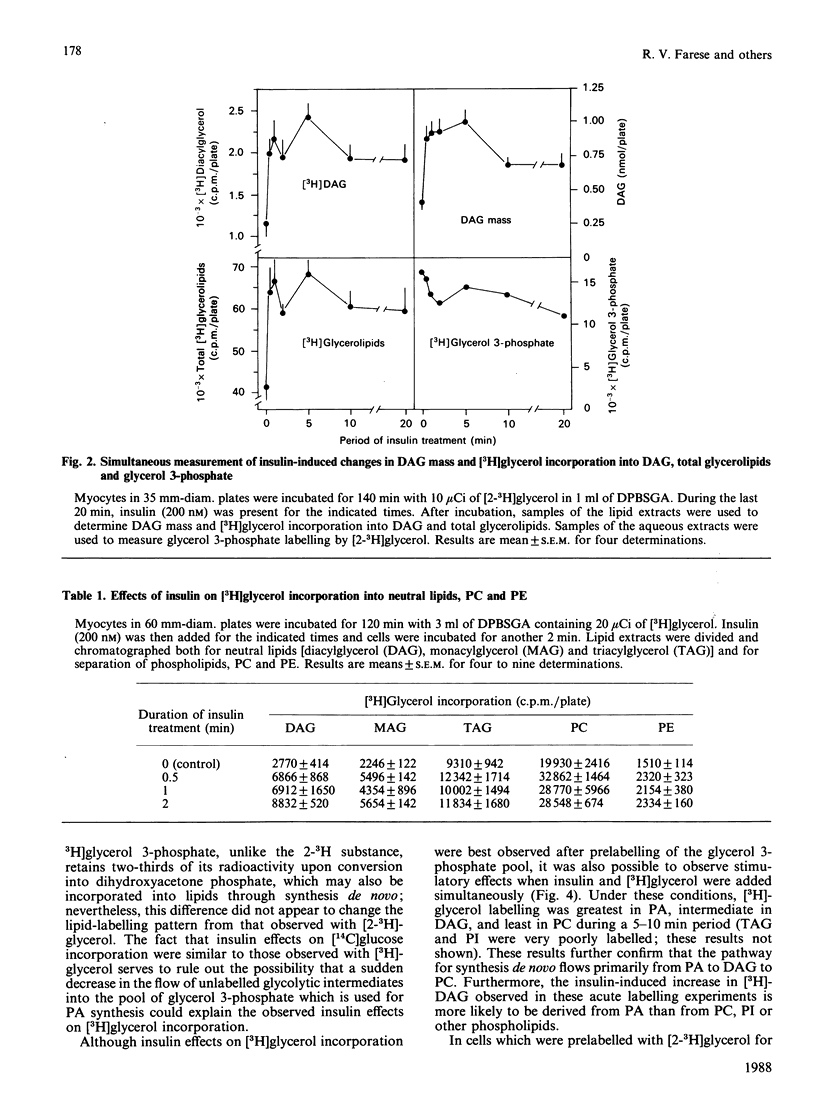
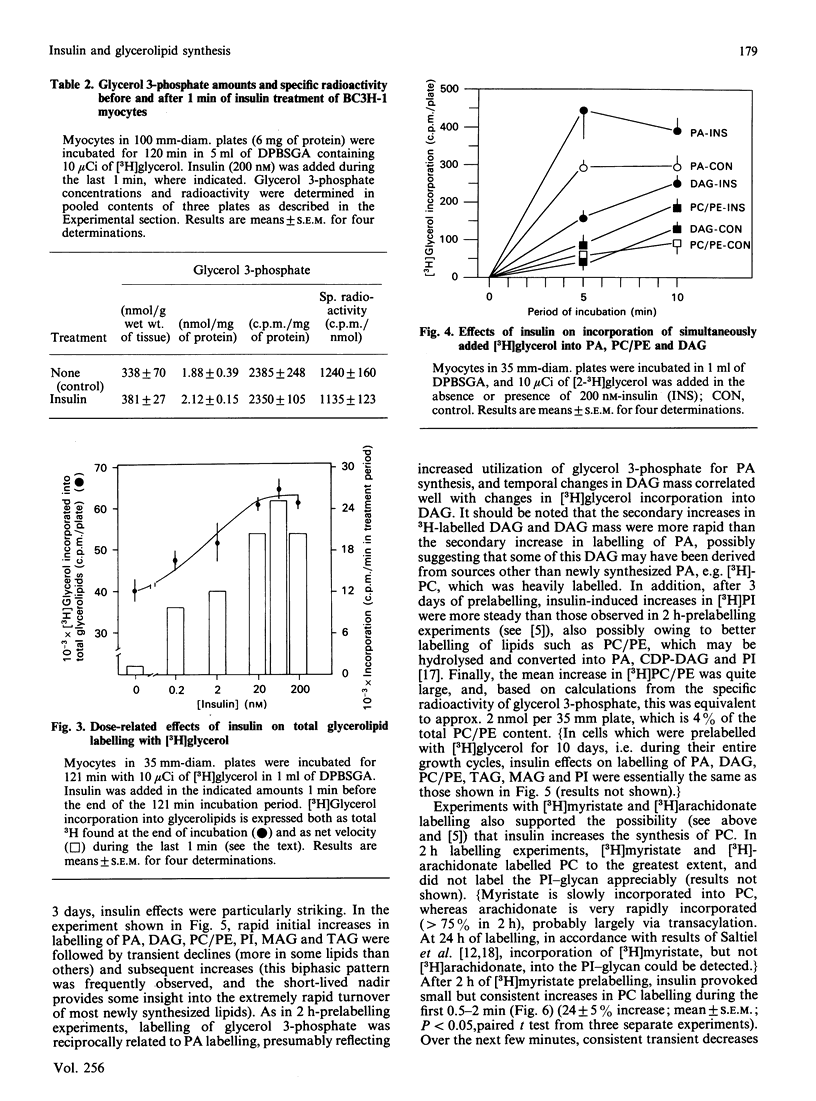
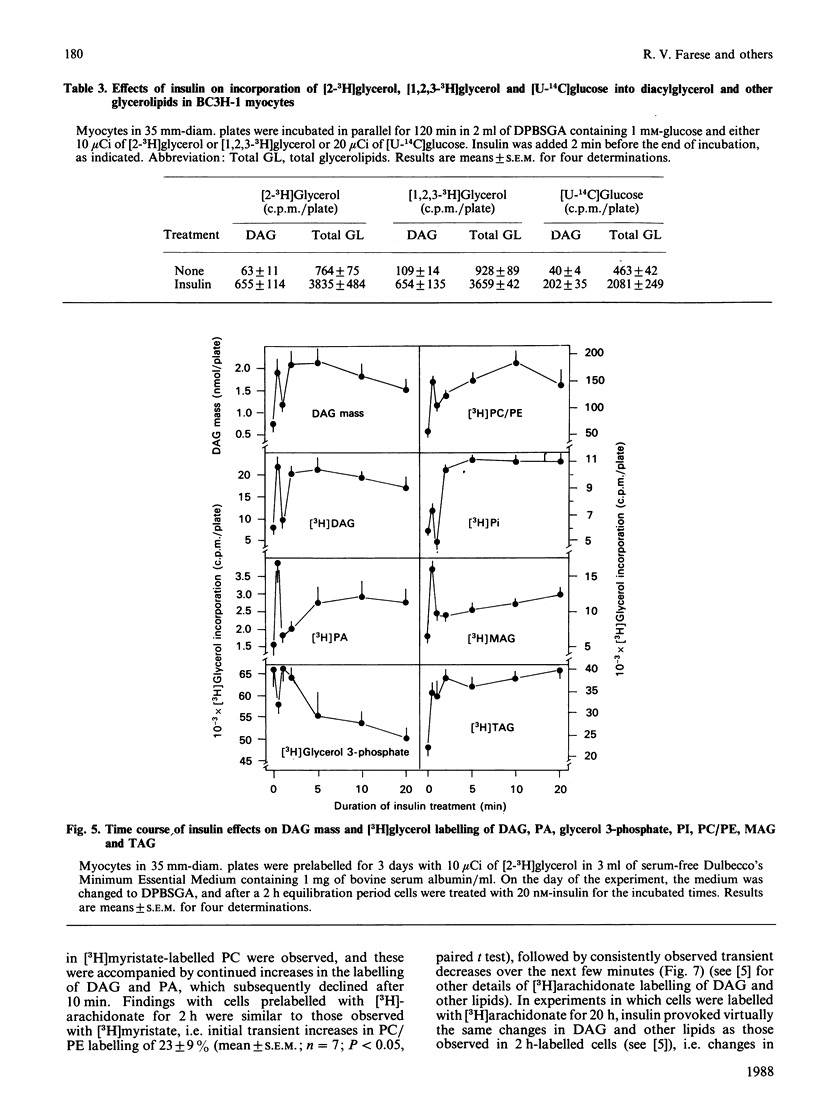
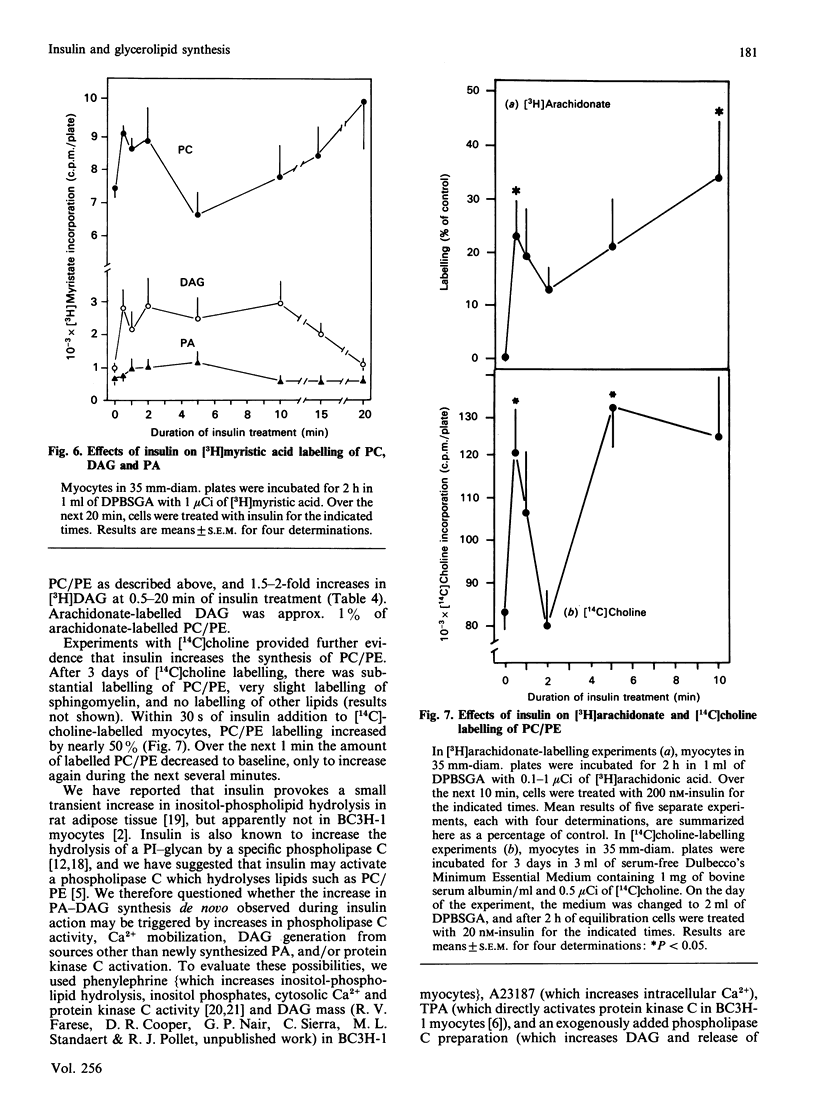
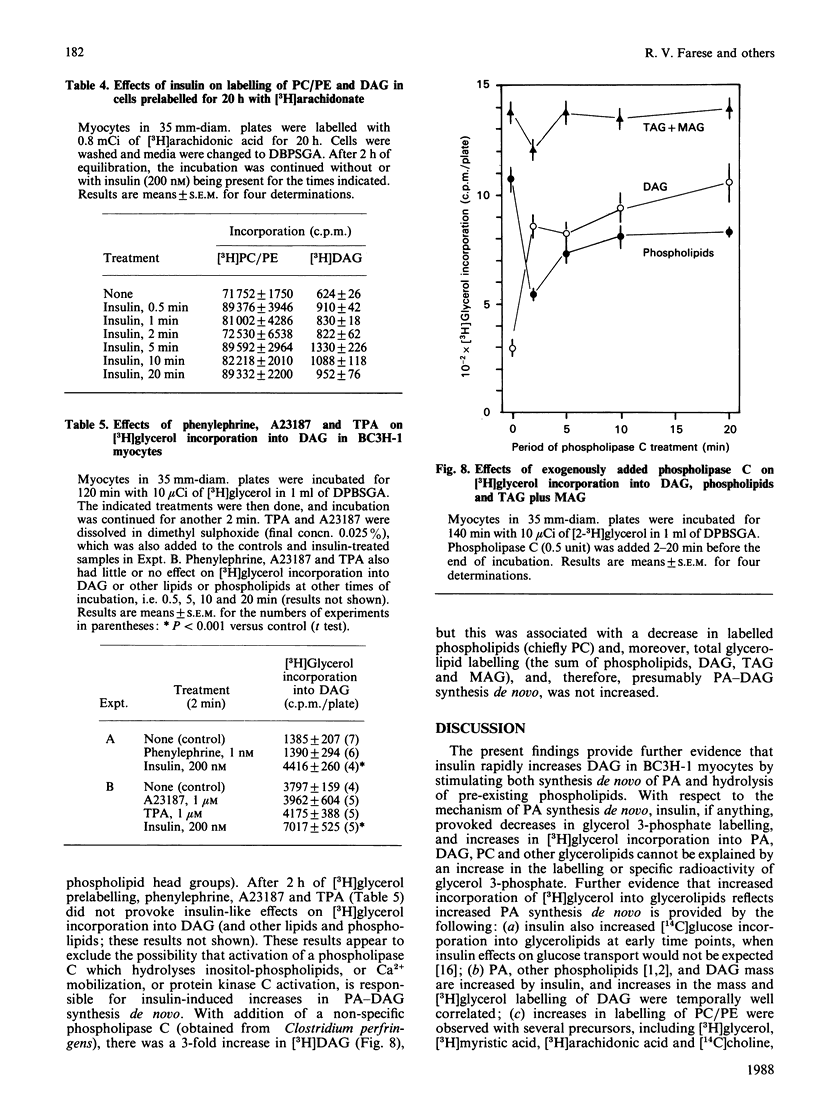
We previously suggested that insulin increases diacylglycerol (DAG) in BC3H-1 myocytes, both by increases in synthesis de novo of phosphatidic acid (PA) and by hydrolysis of non-inositol-containing phospholipids, such as phosphatidylcholine (PC) and phosphatidylethanolamine (PE). We have now evaluated these insulin effects more thoroughly, and several potential mechanisms for their induction. In studies of the effect on PA synthesis de novo, insulin stimulated [2-3H]glycerol incorporation into PA, DAG, PC/PE and total glycerolipids of BC3H-1 myocytes, regardless of whether insulin was added simultaneously with, or after 2 h or 3 or 10 days of prelabelling with, [2-3H]glycerol. In prelabelled cells, time-related changes in [2-3H]glycerol labelling of DAG correlated well with increases in DAG content: both were maximal in 30-60 s and persisted for 20-30 min. [2-3H]Glycerol labelling of glycerol 3-phosphate, on the other hand, was decreased by insulin, presumably reflecting increased utilization for PA synthesis. Glycerol 3-phosphate concentrations were 0.36 and 0.38 mM before and 1 min after insulin treatment, and insulin effects could not be explained by increases in glycerol 3-phosphate specific radioactivity. In addition to that of [2-3H]glycerol, insulin increased [U-14C]glucose and [1,2,3-3H]glycerol incorporation into DAG and other glycerolipids. Effects of insulin on [2-3H]glycerol incorporation into DAG and other glycerolipids were half-maximal and maximal at 2 nM- and 20 nM-insulin respectively, and were not dependent on glucose concentration in the medium, extracellular Ca2+ or protein synthesis. Despite good correlation between [3H]DAG and DAG content, calculated increases in DAG content from glycerol 3-phosphate specific radioactivity (i.e. via the pathway of PA synthesis de novo) could account for only 15-30% of the observed increases in DAG content. In addition to increases in [3H]glycerol labelling of PC/PE, insulin rapidly (within 30 s) increased PC/PE labelling by [3H]arachidonic acid, [3H]myristic acid, and [14C]choline. Phenylephrine, ionophore A23187 and phorbol esters did not increase [2-3H]glycerol incorporation into DAG or other glycerolipids in 2-h-prelabelling experiments; thus activation of the phospholipase C which hydrolyses phosphatidylinositol, its mono- and bis-phosphate, Ca2+ mobilization, and protein kinase C activation, appear to be ruled out as mechanisms to explain the insulin effect on synthesis de novo of PA, DAG and PC.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler S. K., Brown R. D., Taylor P. The relationship between phosphatidylinositol metabolism and mobilization of intracellular calcium elicited by alpha1-adrenergic receptor stimulation in BC3H-1 muscle cells. Mol Pharmacol. 1984 Nov;26(3):405–413. [PubMed] [Google Scholar]
- Cherqui G., Caron M., Wicek D., Lascols O., Capeau J., Picard J. Insulin stimulation of glucose metabolism in rat adipocytes: possible implication of protein kinase C. Endocrinology. 1986 May;118(5):1759–1769. doi: 10.1210/endo-118-5-1759. [DOI] [PubMed] [Google Scholar]
- Cooper D. R., Konda T. S., Standaert M. L., Davis J. S., Pollet R. J., Farese R. V. Insulin increases membrane and cytosolic protein kinase C activity in BC3H-1 myocytes. J Biol Chem. 1987 Mar 15;262(8):3633–3639. [PubMed] [Google Scholar]
- Cooper D. R., de Ruiz Galaretta C. M., Fanjul L. F., Mojsilovic L., Standaert M. L., Pollet R. J., Farese R. V. Insulin but not phorbol ester treatment increases phosphorylation of vinculin by protein kinase C in BC3H-1 myocytes. FEBS Lett. 1987 Apr 6;214(1):122–126. doi: 10.1016/0014-5793(87)80025-x. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J. Measurement of flow of carbon atoms from glucose and glycogen glucose to glyceride glycerol and glycerol in rat heart and epididymal adipose tissue. Effects of insulin, adrenaline and alloxan-diabetes. Biochem J. 1967 Aug;104(2):423–434. doi: 10.1042/bj1040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese R. V., Barnes D. E., Davis J. S., Standaert M. L., Pollet R. J. Effects of insulin and protein synthesis inhibitors on phospholipid metabolism, diacylglycerol levels, and pyruvate dehydrogenase activity in BC3H-1 cultured myocytes. J Biol Chem. 1984 Jun 10;259(11):7094–7100. [PubMed] [Google Scholar]
- Farese R. V., Cooper D. R., Konda T. S., Nair G., Standaert M. L., Pollet R. J. Insulin provokes co-ordinated increases in the synthesis of phosphatidylinositol, phosphatidylinositol phosphates and the phosphatidylinositol-glycan in BC3H-1 myocytes. Biochem J. 1988 Nov 15;256(1):185–188. doi: 10.1042/bj2560185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese R. V., Davis J. S., Barnes D. E., Standaert M. L., Babischkin J. S., Hock R., Rosic N. K., Pollet R. J. The de novo phospholipid effect of insulin is associated with increases in diacylglycerol, but not inositol phosphates or cytosolic Ca2+. Biochem J. 1985 Oct 15;231(2):269–278. doi: 10.1042/bj2310269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese R. V., Konda T. S., Davis J. S., Standaert M. L., Pollet R. J., Cooper D. R. Insulin rapidly increases diacylglycerol by activating de novo phosphatidic acid synthesis. Science. 1987 May 1;236(4801):586–589. doi: 10.1126/science.3107122. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Kuo J. Y., Babischkin J. S., Davis J. S. Insulin provokes a transient activation of phospholipase C in the rat epididymal fat pad. J Biol Chem. 1986 Jul 5;261(19):8589–8592. [PubMed] [Google Scholar]
- Farese R. V., Larson R. E., Sabir M. A. Insulin acutely increases phospholipids in the phosphatidate-inositide cycle in rat adipose tissue. J Biol Chem. 1982 Apr 25;257(8):4042–4045. [PubMed] [Google Scholar]
- Farese R. V., Rosic N., Standaert M., Babischkin J., Cooper D. R., Davis J. S., Pollet R. J. Further evidence implicating diacylglycerol generation and protein kinase C activation in agonist-induced increases in glucose uptake. Insulin-like effects of phenylephrine in BC3H-1 myocytes. Diabetes. 1986 Sep;35(9):951–957. doi: 10.2337/diab.35.9.951. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Standaert M. L., Barnes D. E., Davis J. S., Pollet R. J. Phorbol ester provokes insulin-like effects on glucose transport, amino acid uptake, and pyruvate dehydrogenase activity in BC3H-1 cultured myocytes. Endocrinology. 1985 Jun;116(6):2650–2655. doi: 10.1210/endo-116-6-2650. [DOI] [PubMed] [Google Scholar]
- Kirsch D., Obermaier B., Häring H. U. Phorbolesters enhance basal D-glucose transport but inhibit insulin stimulation of D-glucose transport and insulin binding in isolated rat adipocytes. Biochem Biophys Res Commun. 1985 Apr 30;128(2):824–832. doi: 10.1016/0006-291x(85)90121-4. [DOI] [PubMed] [Google Scholar]
- Pennington S. R., Martin B. R. Insulin-stimulated phosphoinositide metabolism in isolated fat cells. J Biol Chem. 1985 Sep 15;260(20):11039–11045. [PubMed] [Google Scholar]
- Pershadsingh H. A., Shade D. L., McDonald J. M. Insulin-dependent alterations of phorbol ester binding to adipocyte subcellular constituents. Evidence for the involvement of protein kinase C in insulin action. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1384–1389. doi: 10.1016/0006-291x(87)91591-9. [DOI] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Saltiel A. R., Fox J. A., Sherline P., Cuatrecasas P. Insulin-stimulated hydrolysis of a novel glycolipid generates modulators of cAMP phosphodiesterase. Science. 1986 Aug 29;233(4767):967–972. doi: 10.1126/science.3016898. [DOI] [PubMed] [Google Scholar]
- Saltiel A. R., Sherline P., Fox J. A. Insulin-stimulated diacylglycerol production results from the hydrolysis of a novel phosphatidylinositol glycan. J Biol Chem. 1987 Jan 25;262(3):1116–1121. [PubMed] [Google Scholar]
- Standaert M. L., Schimmel S. D., Pollet R. J. The development of insulin receptors and responses in the differentiating nonfusing muscle cell line BC3H-1. J Biol Chem. 1984 Feb 25;259(4):2337–2345. [PubMed] [Google Scholar]
- Stevens E. V., Husbands D. R. Stimulation of rat liver glycerol-3-phosphate acyltransferase activity by acid- and heat-stable low-molecular-weight substances from skeletal muscle of rats treated with insulin. Arch Biochem Biophys. 1987 Nov 1;258(2):361–364. doi: 10.1016/0003-9861(87)90356-0. [DOI] [PubMed] [Google Scholar]
- Walaas S. I., Horn R. S., Adler A., Albert K. A., Walaas O. Insulin increases membrane protein kinase C activity in rat diaphragm. FEBS Lett. 1987 Aug 17;220(2):311–318. doi: 10.1016/0014-5793(87)80837-2. [DOI] [PubMed] [Google Scholar]
- van de Werve G., Zaninetti D., Lang U., Vallotton M. B., Jeanrenaud B. Identification of a major defect in insulin-resistant tissues of genetically obese (fa/fa) rats. Impaired protein kinase C. Diabetes. 1987 Mar;36(3):310–314. doi: 10.2337/diab.36.3.310. [DOI] [PubMed] [Google Scholar]


