Abstract
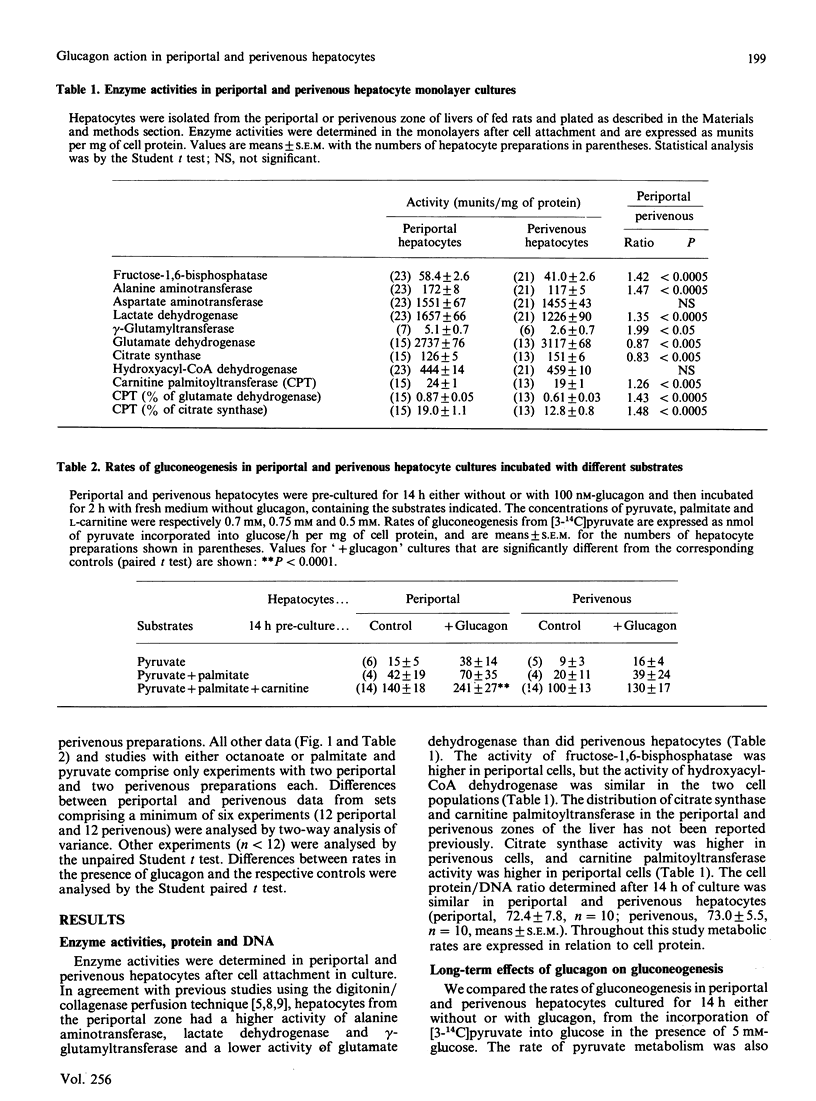
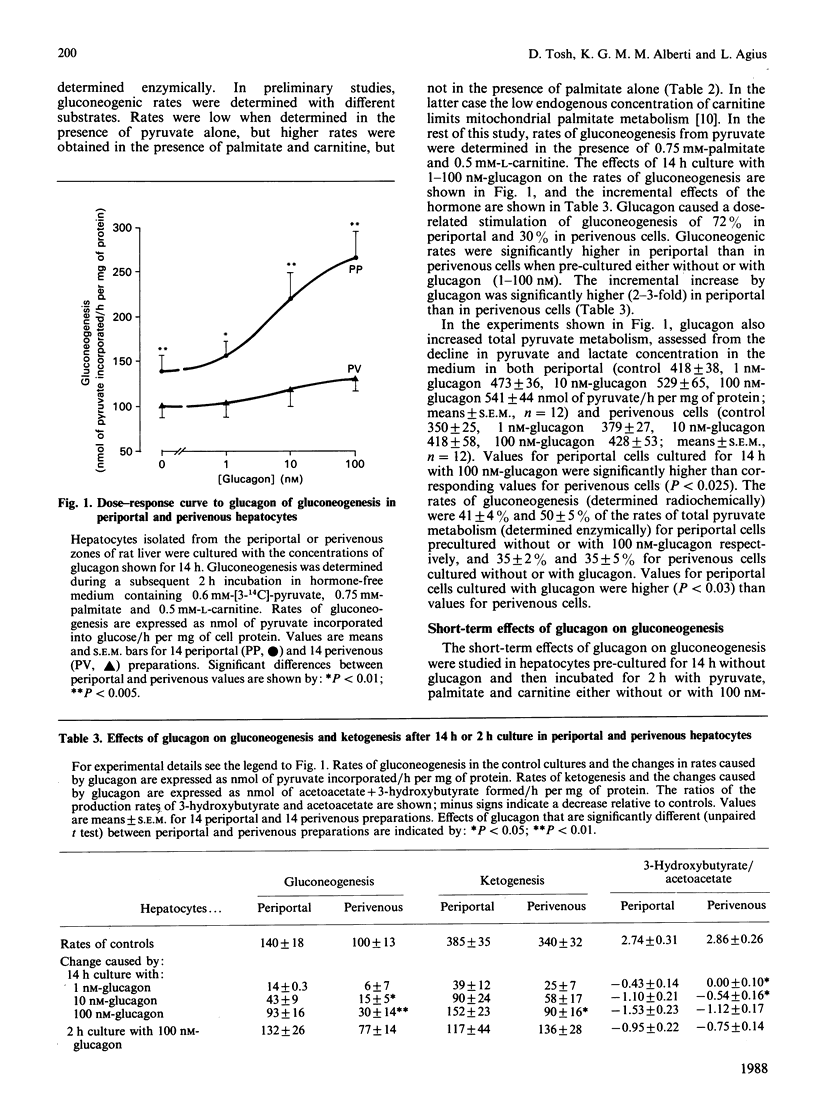
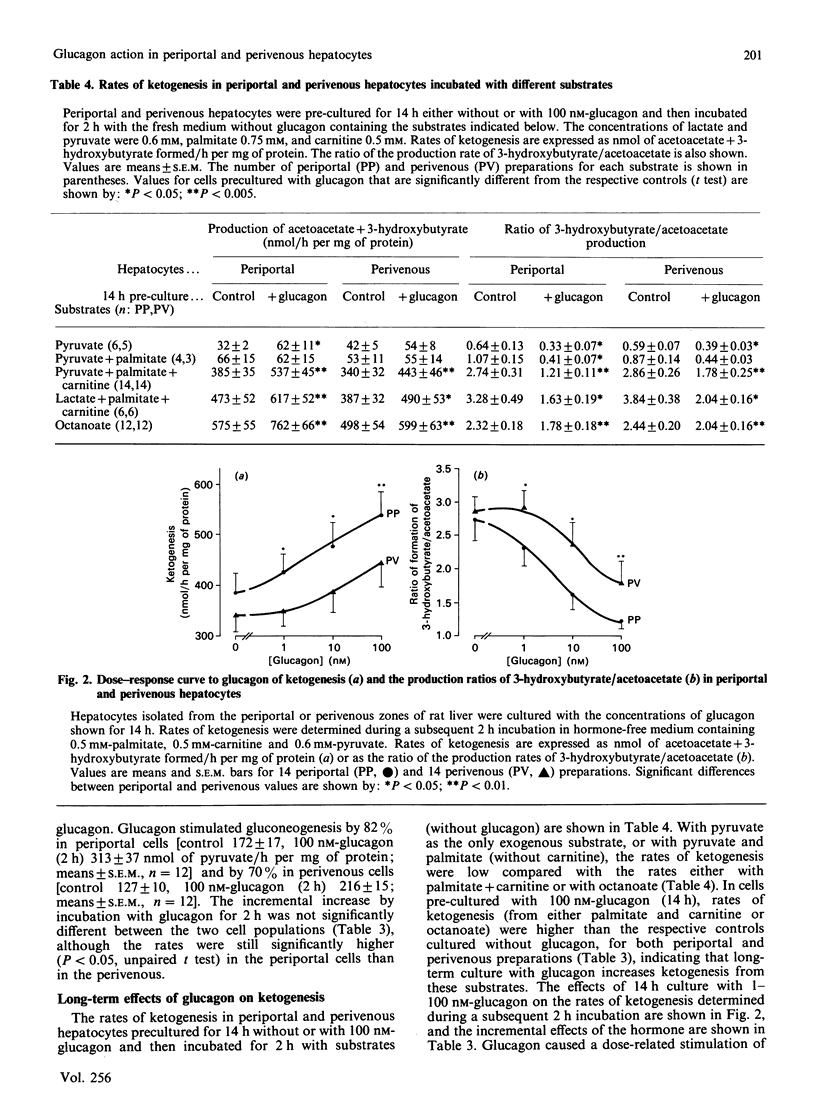
Hepatocytes isolated from the periportal or perivenous zones of livers of fed rats were used to study the long-term (14 h) and short-term (2 h) effects of glucagon on gluconeogenesis and ketogenesis. Long-term culture with glucagon (100 nM) resulted in a greater increase (P less than 0.01) in gluconeogenesis in periportal than in perivenous cells (93 +/- 16 versus 30 +/- 14 nmol/h per mg of protein; 72% versus 30% increase), but short-term incubation (2 h) with glucagon resulted in similar stimulation in the two cell populations. Rates of ketogenesis (acetoacetate and D-3-hydroxybutyrate production) were not significantly higher in periportal cells cultured without glucagon, compared with perivenous cells. However, after long-term culture with glucagon, the periportal cells had a significantly higher rate of ketogenesis (from either palmitate or octanoate as substrate), but a lower 3-hydroxybutyrate/acetoacetate production ratio, suggesting a more oxidized mitochondrial NADH/NAD+ redox state despite the higher rate of beta-oxidation. Periportal hepatocytes had a higher activity of carnitine palmitoyltransferase but a lower activity of citrate synthase than did perivenous cells. These findings suggest that: (i) glucagon elicits greater long-term stimulation of gluconeogenesis in periportal than in perivenous hepatocytes maintained in culture; (ii) after culture with glucagon, the rates of ketogenesis and the mitochondrial redox state differ in periportal and perivenous hepatocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agius L., Alberti K. G. Regulation of flux through pyruvate dehydrogenase and pyruvate carboxylase in rat hepatocytes. Effects of fatty acids and glucagon. Eur J Biochem. 1985 Nov 4;152(3):699–707. doi: 10.1111/j.1432-1033.1985.tb09250.x. [DOI] [PubMed] [Google Scholar]
- Agius L., Chowdhury M. H., Alberti K. G. Regulation of ketogenesis, gluconeogenesis and the mitochondrial redox state by dexamethasone in hepatocyte monolayer cultures. Biochem J. 1986 Nov 1;239(3):593–601. doi: 10.1042/bj2390593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agius L., Chowdhury M. H., Davis S. N., Alberti K. G. Regulation of ketogenesis, gluconeogenesis, and glycogen synthesis by insulin and proinsulin in rat hepatocyte monolayer cultures. Diabetes. 1986 Nov;35(11):1286–1293. doi: 10.2337/diab.35.11.1286. [DOI] [PubMed] [Google Scholar]
- Agius L., Wright P. D., Alberti K. G. Carnitine acyltransferases and acyl-CoA hydrolases in human and rat liver. Clin Sci (Lond) 1987 Jul;73(1):3–10. doi: 10.1042/cs0730003. [DOI] [PubMed] [Google Scholar]
- Brdiczka D., Pette D., Brunner G., Miller F. Kompartimentierte Verteilung von Enzymen in Rattenlebermitochondrien. Eur J Biochem. 1968 Jul;5(2):294–304. doi: 10.1111/j.1432-1033.1968.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Christiansen R. Z. Regulation of palmitate metabolism by carnitine and glucagon in hepatocytes isolated from fasted and carbohydrate refed rats. Biochim Biophys Acta. 1977 Aug 24;488(2):249–262. doi: 10.1016/0005-2760(77)90182-5. [DOI] [PubMed] [Google Scholar]
- Gumucio J. J., Miller D. L. Functional implications of liver cell heterogeneity. Gastroenterology. 1981 Feb;80(2):393–403. [PubMed] [Google Scholar]
- Ji S., Lemasters J. J., Thurman R. G. A non-invasive method to study metabolic events within sublobular regions of hemoglobin-free perfused liver. FEBS Lett. 1980 Apr 21;113(1):37–42. doi: 10.1016/0014-5793(80)80489-3. [DOI] [PubMed] [Google Scholar]
- Jungermann K. Metabolic zonation of liver parenchyma: significance for the regulation of glycogen metabolism, gluconeogenesis, and glycolysis. Diabetes Metab Rev. 1987 Jan;3(1):269–293. doi: 10.1002/dmr.5610030112. [DOI] [PubMed] [Google Scholar]
- Katz N. R., Fischer W., Giffhorn S. Distribution of enzymes of fatty acid and ketone body metabolism in periportal and perivenous rat-liver tissue. Eur J Biochem. 1983 Sep 1;135(1):103–107. doi: 10.1111/j.1432-1033.1983.tb07623.x. [DOI] [PubMed] [Google Scholar]
- Katz N. R., Fischer W., Ick M. Heterogeneous distribution of ATP citrate lyase in rat-liver parenchyma. Microradiochemical determination in microdissected periportal and perivenous liver tissue. Eur J Biochem. 1983 Feb 1;130(2):297–301. doi: 10.1111/j.1432-1033.1983.tb07151.x. [DOI] [PubMed] [Google Scholar]
- Katz N., Teutsch H. F., Jungermann K., Sasse D. Heterogeneous reciprocal localization of fructose-1,6-bisphosphatase and of glucokinase in microdissected periportal and perivenous rat liver tissue. FEBS Lett. 1977 Nov 15;83(2):272–276. doi: 10.1016/0014-5793(77)81021-1. [DOI] [PubMed] [Google Scholar]
- Kera Y., Sippel H. W., Penttilä K. E., Lindros K. O. Acinar distribution of glutathione-dependent detoxifying enzymes. Low glutathione peroxidase activity in perivenous hepatocytes. Biochem Pharmacol. 1987 Jun 15;36(12):2003–2006. doi: 10.1016/0006-2952(87)90500-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Pecq J. B., Paoletti C. A new fluorometric method for RNA and DNA determination. Anal Biochem. 1966 Oct;17(1):100–107. doi: 10.1016/0003-2697(66)90012-1. [DOI] [PubMed] [Google Scholar]
- Lindros K. O., Penttilä K. E. Digitonin-collagenase perfusion for efficient separation of periportal or perivenous hepatocytes. Biochem J. 1985 Jun 15;228(3):757–760. doi: 10.1042/bj2280757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVIKOFF A. B. Cell heterogeneity within the hepatic lobule of the rat: staining reactions. J Histochem Cytochem. 1959 Jul;7(4):240–244. doi: 10.1177/7.4.240. [DOI] [PubMed] [Google Scholar]
- Olson M. J., Handler J. A., Thurman R. G. Mechanism of zone-specific hepatic steatosis caused by valproate: inhibition of ketogenesis in periportal regions of the liver lobule. Mol Pharmacol. 1986 Dec;30(6):520–525. [PubMed] [Google Scholar]
- Olson M. J., Thurman R. G. Quantitation of ketogenesis in periportal and pericentral regions of the liver lobule. Arch Biochem Biophys. 1987 Feb 15;253(1):26–37. doi: 10.1016/0003-9861(87)90633-3. [DOI] [PubMed] [Google Scholar]
- Pösö A. R., Penttilä K. E., Suolinna E. M., Lindros K. O. Urea synthesis in freshly isolated and in cultured periportal and perivenous hepatocytes. Biochem J. 1986 Oct 15;239(2):263–267. doi: 10.1042/bj2390263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistorff B., Dich J., Grunnet N. Periportal and perivenous hepatocytes retain their zonal characteristics in primary culture. Biochem Biophys Res Commun. 1986 Sep 30;139(3):1055–1061. doi: 10.1016/s0006-291x(86)80284-4. [DOI] [PubMed] [Google Scholar]
- Quistorff B. Digitonin perfusion in the study of metabolic zonation of the rat liver: potassium as an intracellular concentration reference. Biochem Soc Trans. 1987 Jun;15(3):361–363. doi: 10.1042/bst0150361. [DOI] [PubMed] [Google Scholar]
- Quistorff B. Gluconeogenesis in periportal and perivenous hepatocytes of rat liver, isolated by a new high-yield digitonin/collagenase perfusion technique. Biochem J. 1985 Jul 1;229(1):221–226. doi: 10.1042/bj2290221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse D., Katz N., Jungermann K. Functional heterogeneity of rat liver parenchyma and of isolated hepatocytes. FEBS Lett. 1975 Sep 1;57(1):83–88. doi: 10.1016/0014-5793(75)80157-8. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer M., Pette D. Microphotometric studies on intraacinar enzyme distribution in rat liver. Histochemistry. 1979 Nov;64(1):23–33. doi: 10.1007/BF00493352. [DOI] [PubMed] [Google Scholar]
- Yamazaki R. K. Glucagon stimulation of mitochondrial respiration. J Biol Chem. 1975 Oct 10;250(19):7924–7930. [PubMed] [Google Scholar]


