Abstract
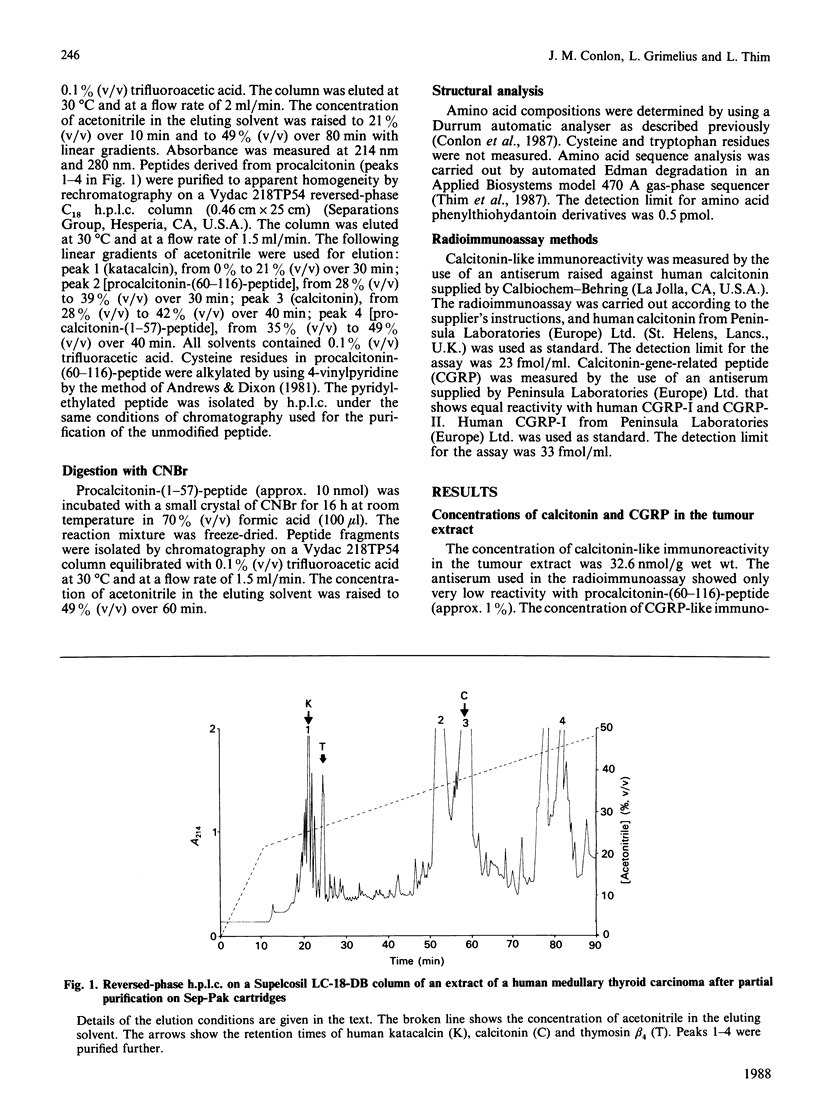
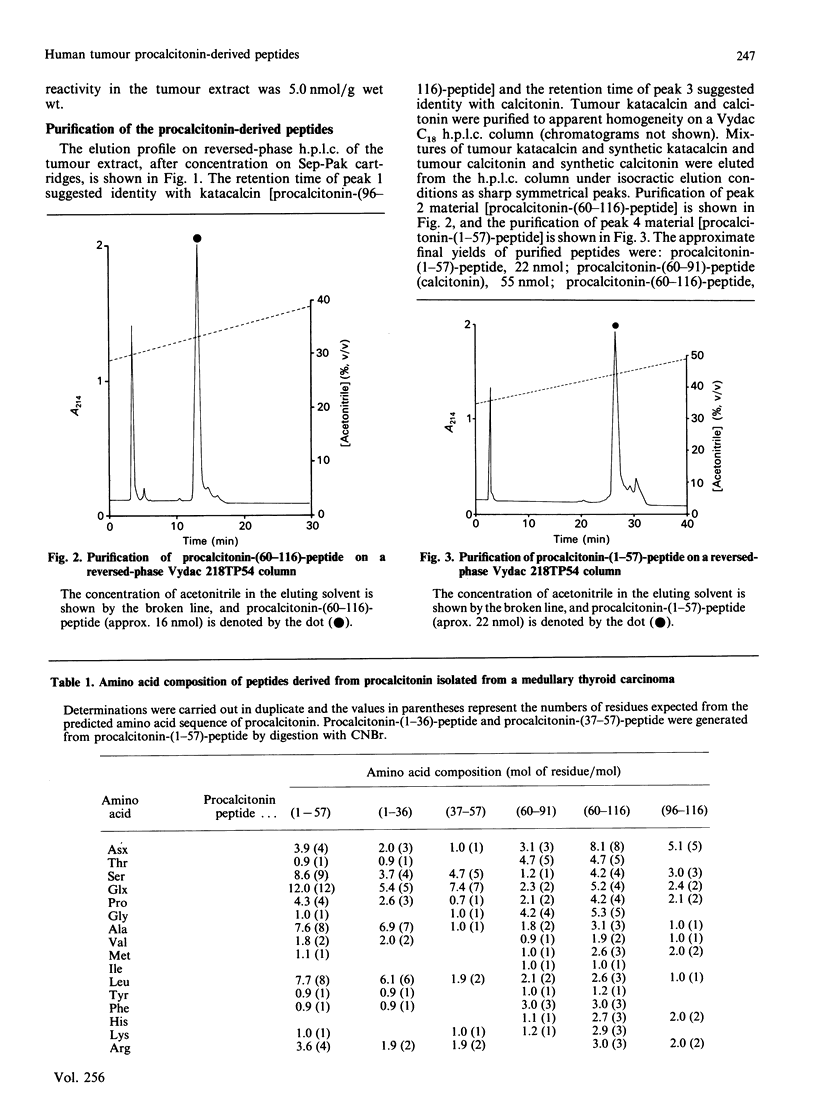
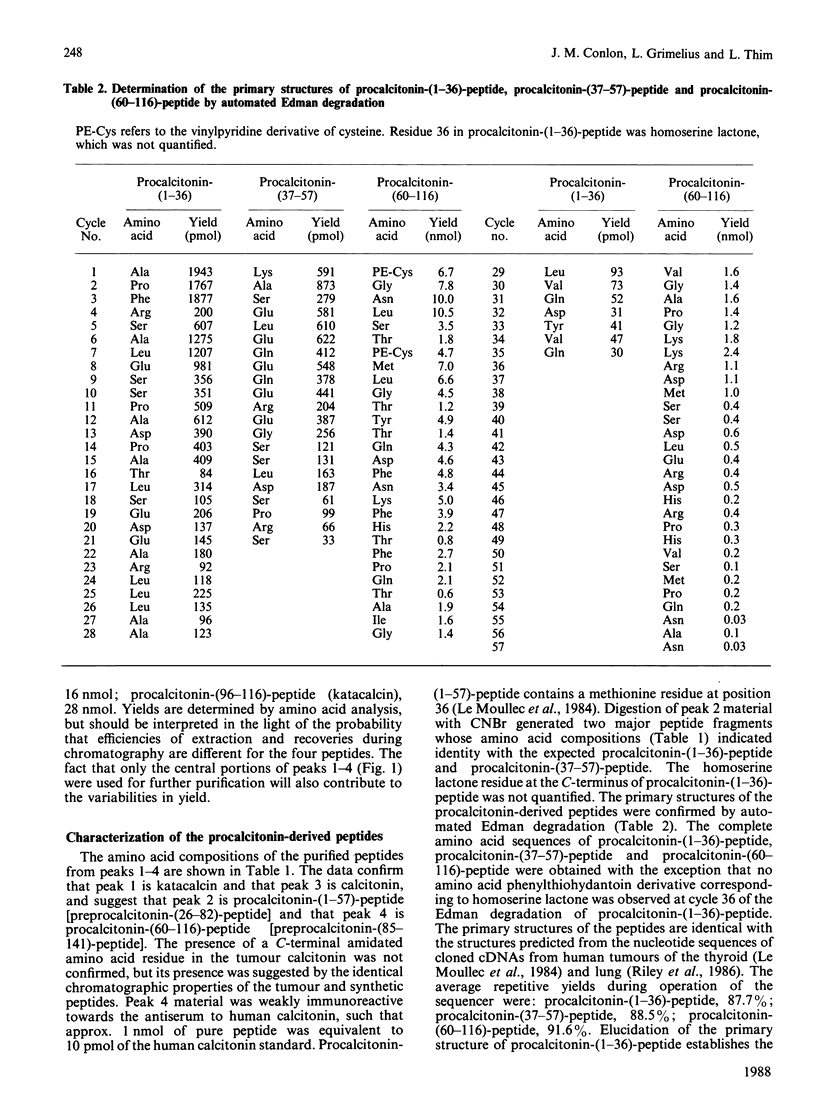
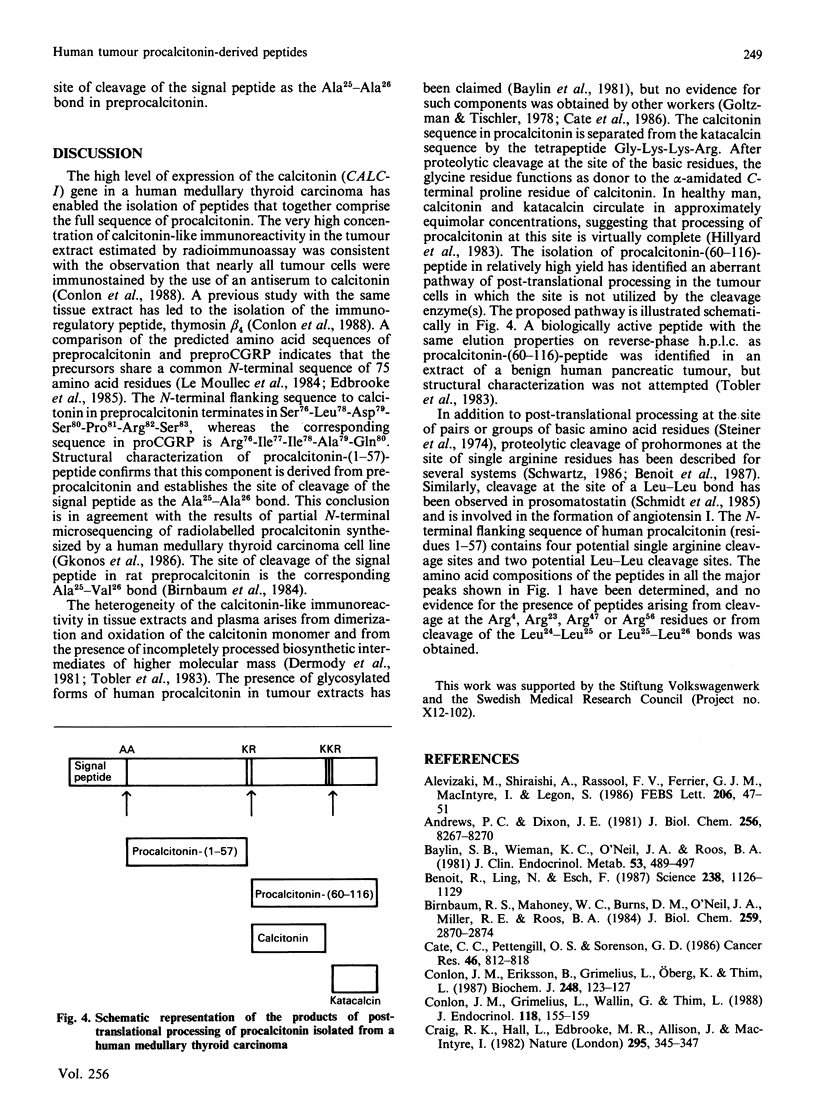
Four peptides derived from procalcitonin were isolated in high yield from an extract of a human medullary thyroid carcinoma. The peptides were identified as procalcitonin-(1-57)-peptide, procalcitonin-(60-91)-peptide (calcitonin), procalcitonin-(60-116)-peptide and procalcitonin-(96-116)-peptide (katacalcin). Determination of the amino acid sequence of procalcitonin-(1-57)-peptide has demonstrated that the Ala25-Ala26 bond in preprocalcitonin is the site of cleavage of the signal peptide. Procalcitonin-(60-116)-peptide represents calcitonin extended from its C-terminus by the sequence Gly-Lys-Lys-Arg-katacalcin, and its formation is indicative of an aberrant pathway of procalcitonin processing in the tumour cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alevizaki M., Shiraishi A., Rassool F. V., Ferrier G. J., MacIntyre I., Legon S. The calcitonin-like sequence of the beta CGRP gene. FEBS Lett. 1986 Sep 29;206(1):47–52. doi: 10.1016/0014-5793(86)81338-2. [DOI] [PubMed] [Google Scholar]
- Andrews P. C., Dixon J. E. Isolation and structure of a peptide hormone predicted from a mRNA sequence. A second somatostatin from the catfish pancreas. J Biol Chem. 1981 Aug 25;256(16):8267–8270. [PubMed] [Google Scholar]
- Baylin S. B., Wieman K. C., O'Neil J. A., Roos B. A. Multiple forms of human tumor calcitonin demonstrated by denaturing polyacrylamide gel electrophoresis and lectin affinity chromatography. J Clin Endocrinol Metab. 1981 Sep;53(3):489–497. doi: 10.1210/jcem-53-3-489. [DOI] [PubMed] [Google Scholar]
- Benoit R., Ling N., Esch F. A new prosomatostatin-derived peptide reveals a pattern for prohormone cleavage at monobasic sites. Science. 1987 Nov 20;238(4830):1126–1129. doi: 10.1126/science.2891188. [DOI] [PubMed] [Google Scholar]
- Birnbaum R. S., Mahoney W. C., Burns D. M., O'Neil J. A., Miller R. E., Roos B. A. Identification of procalcitonin in a rat medullary thyroid carcinoma cell line. J Biol Chem. 1984 Mar 10;259(5):2870–2874. [PubMed] [Google Scholar]
- Cate C. C., Pettengill O. S., Sorenson G. D. Biosynthesis of procalcitonin in small cell carcinoma of the lung. Cancer Res. 1986 Feb;46(2):812–818. [PubMed] [Google Scholar]
- Conlon J. M., Eriksson B., Grimelius L., Oberg K., Thim L. Characterization of three peptides derived from prosomatostatin [prosomatostatin-(1-63)-, -(65-76)- and -(79-92)-peptides] in a human pancreatic tumour. Biochem J. 1987 Nov 15;248(1):123–127. doi: 10.1042/bj2480123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon J. M., Grimelius L., Wallin G., Thim L. Isolation and structural characterization of thymosin-beta 4 from a human medullary thyroid carcinoma. J Endocrinol. 1988 Jul;118(1):155–159. doi: 10.1677/joe.0.1180155. [DOI] [PubMed] [Google Scholar]
- Craig R. K., Hall L., Edbrooke M. R., Allison J., MacIntyre I. Partial nucleotide sequence of human calcitonin precursor mRNA identifies flanking cryptic peptides. Nature. 1982 Jan 28;295(5847):345–347. doi: 10.1038/295345a0. [DOI] [PubMed] [Google Scholar]
- Dermody W. C., Rosen M. A., Ananthaswamy R., McCormick W. M., Levy A. G. Characterization of the major forms of human calcitonin in tissue and serum. J Clin Endocrinol Metab. 1981 Jun;52(6):1090–1098. doi: 10.1210/jcem-52-6-1090. [DOI] [PubMed] [Google Scholar]
- Desplan C., Benicourt C., Jullienne A., Segond N., Calmettes C., Moukhtar M. S., Milhaud G. Cell free translation of mRNA coding for human and murine calcitonin. FEBS Lett. 1980 Aug 11;117(1):89–92. doi: 10.1016/0014-5793(80)80919-7. [DOI] [PubMed] [Google Scholar]
- Edbrooke M. R., Parker D., McVey J. H., Riley J. H., Sorenson G. D., Pettengill O. S., Craig R. K. Expression of the human calcitonin/CGRP gene in lung and thyroid carcinoma. EMBO J. 1985 Mar;4(3):715–724. doi: 10.1002/j.1460-2075.1985.tb03688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmiche J. P., Chayvialle J. A., Dubois P. M., David L., Descos F., Paulin C., Ducastelle T., Colin R., Geffroy Y. Calcitonin-producing pancreatic somatostatinoma. Gastroenterology. 1980 Jun;78(6):1577–1583. [PubMed] [Google Scholar]
- Gkonos P. J., Born W., Jones B. N., Petermann J. B., Keutmann H. T., Birnbaum R. S., Fischer J. A., Roos B. A. Biosynthesis of calcitonin gene-related peptide and calcitonin by a human medullary thyroid carcinoma cell line. J Biol Chem. 1986 Nov 5;261(31):14386–14391. [PubMed] [Google Scholar]
- Goltzman D., Tischler A. S. Characterization of the immunochemical forms of calcitonin released by a medullary thyroid carcinoma in tissue culture. J Clin Invest. 1978 Feb;61(2):449–458. doi: 10.1172/JCI108956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas V., Lin C. R., Kawashima E., Semon D., Swanson L. W., Mermod J. J., Evans R. M., Rosenfeld M. G. Alternative RNA processing events in human calcitonin/calcitonin gene-related peptide gene expression. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1994–1998. doi: 10.1073/pnas.82.7.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moullec J. M., Jullienne A., Chenais J., Lasmoles F., Guliana J. M., Milhaud G., Moukhtar M. S. The complete sequence of human preprocalcitonin. FEBS Lett. 1984 Feb 13;167(1):93–97. doi: 10.1016/0014-5793(84)80839-x. [DOI] [PubMed] [Google Scholar]
- Lumsden J., Ham J., Ellison M. L. Purification and partial characterization of high-molecular-weight forms of ectopic calcitonin from a human bronchial carcinoma cell line. Biochem J. 1980 Oct 1;191(1):239–246. doi: 10.1042/bj1910239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris H. R., Panico M., Etienne T., Tippins J., Girgis S. I., MacIntyre I. Isolation and characterization of human calcitonin gene-related peptide. Nature. 1984 Apr 19;308(5961):746–748. doi: 10.1038/308746a0. [DOI] [PubMed] [Google Scholar]
- Riley J. H., Edbrooke M. R., Craig R. K. Ectopic synthesis of high-Mr calcitonin by the BEN lung carcinoma cell line reflects aberrant proteolytic processing. FEBS Lett. 1986 Mar 17;198(1):71–79. doi: 10.1016/0014-5793(86)81187-5. [DOI] [PubMed] [Google Scholar]
- Schmidt W. E., Mutt V., Kratzin H., Carlquist M., Conlon J. M., Creutzfeldt W. Isolation and characterization of proSS1-32, a peptide derived from the N-terminal region of porcine preprosomatostatin. FEBS Lett. 1985 Nov 11;192(1):141–146. doi: 10.1016/0014-5793(85)80060-0. [DOI] [PubMed] [Google Scholar]
- Schwartz T. W. The processing of peptide precursors. 'Proline-directed arginyl cleavage' and other monobasic processing mechanisms. FEBS Lett. 1986 May 5;200(1):1–10. doi: 10.1016/0014-5793(86)80500-2. [DOI] [PubMed] [Google Scholar]
- Steenbergh P. H., Höppener J. W., Zandberg J., Van de Ven W. J., Jansz H. S., Lips C. J. Calcitonin gene related peptide coding sequence is conserved in the human genome and is expressed in medullary thyroid carcinoma. J Clin Endocrinol Metab. 1984 Aug;59(2):358–360. doi: 10.1210/jcem-59-2-358. [DOI] [PubMed] [Google Scholar]
- Steenbergh P. H., Höppener J. W., Zandberg J., Visser A., Lips C. J., Jansz H. S. Structure and expression of the human calcitonin/CGRP genes. FEBS Lett. 1986 Dec 1;209(1):97–103. doi: 10.1016/0014-5793(86)81091-2. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Kemmler W., Tager H. S., Peterson J. D. Proteolytic processing in the biosynthesis of insulin and other proteins. Fed Proc. 1974 Oct;33(10):2105–2115. [PubMed] [Google Scholar]
- Thim L., Hansen M. T., Sørensen A. R. Secretion of human insulin by a transformed yeast cell. FEBS Lett. 1987 Feb 23;212(2):307–312. doi: 10.1016/0014-5793(87)81366-2. [DOI] [PubMed] [Google Scholar]
- Tobler P. H., Dambacher M. A., Born W., Heitz P. U., Maier R., Fischer J. A. A new bioactive form of human calcitonin. Cancer Res. 1983 Aug;43(8):3793–3799. [PubMed] [Google Scholar]
- Tschopp F. A., Tobler P. H., Fischer J. A. Calcitonin gene-related peptide in the human thyroid, pituitary and brain. Mol Cell Endocrinol. 1984 Jun;36(1-2):53–57. doi: 10.1016/0303-7207(84)90084-4. [DOI] [PubMed] [Google Scholar]
- Zajac J. D., Martin T. J., Hudson P., Niall H., Jacobs J. W. Biosynthesis of calcitonin by human lung cancer cells. Endocrinology. 1985 Feb;116(2):749–755. doi: 10.1210/endo-116-2-749. [DOI] [PubMed] [Google Scholar]


