Abstract
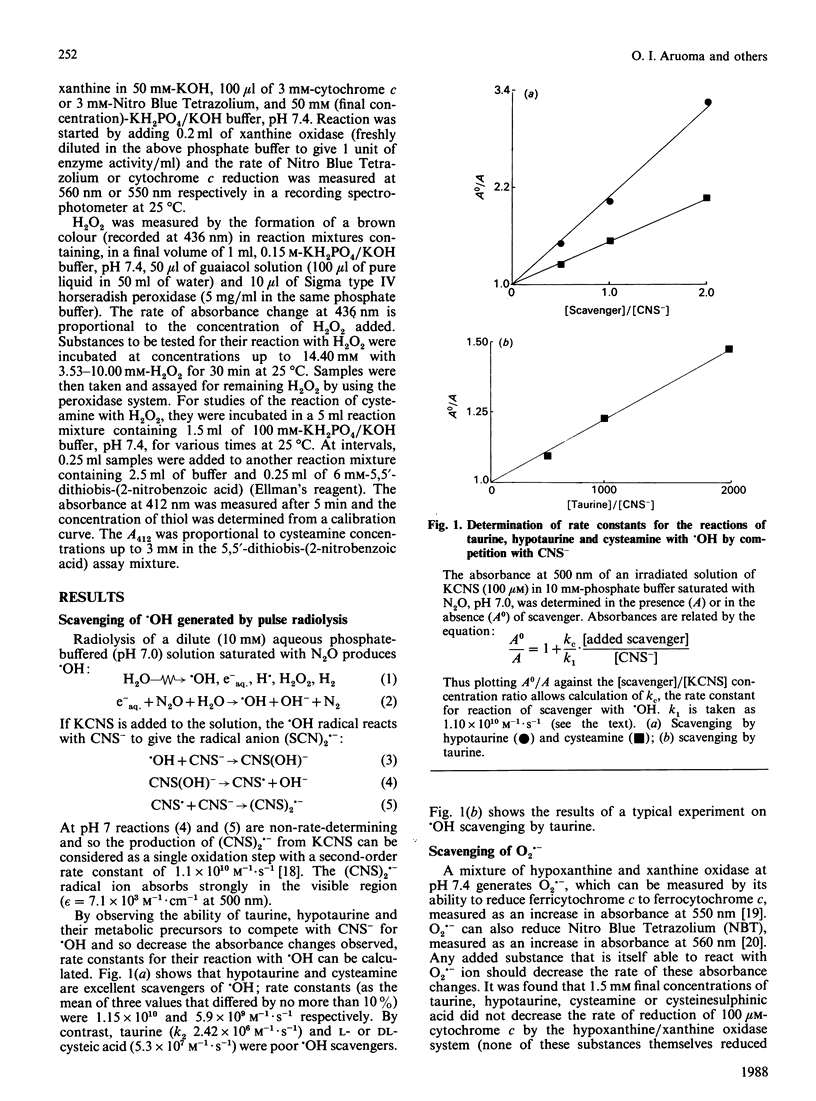
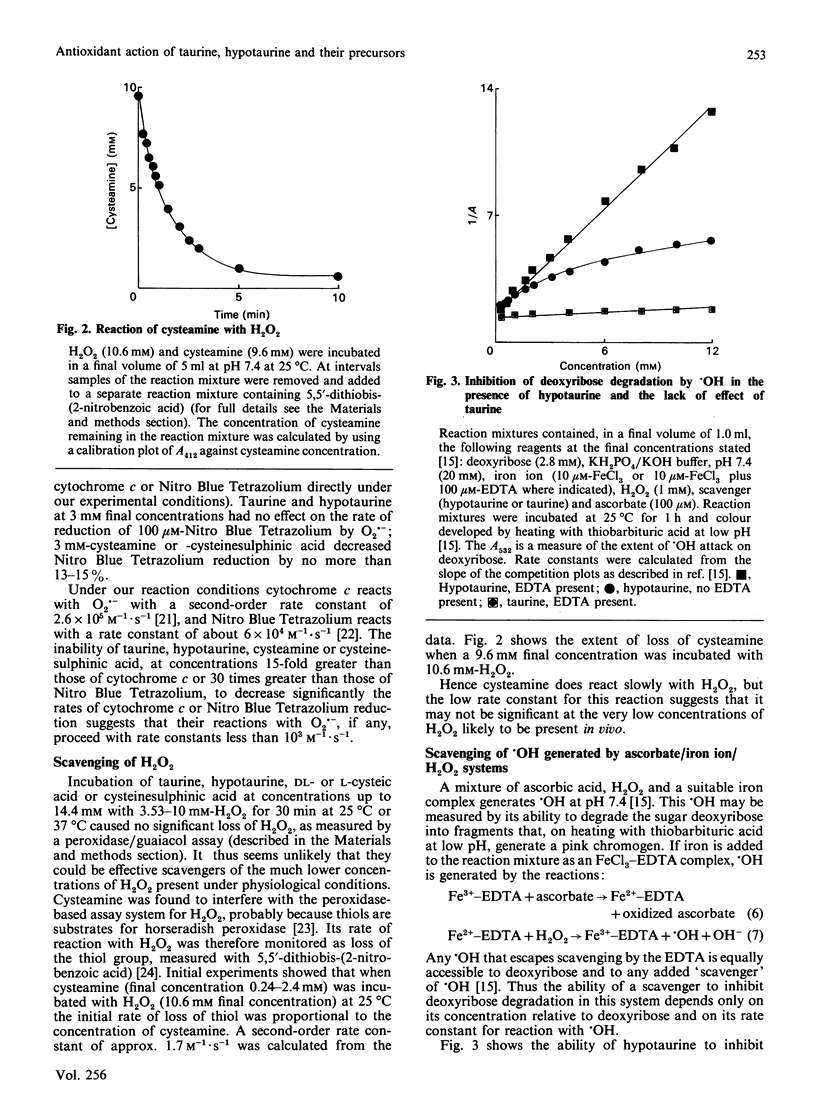
It has been suggested that taurine, hypotaurine and their metabolic precursors (cysteic acid, cysteamine and cysteinesulphinic acid) might act as antioxidants in vivo. The rates of their reactions with the biologically important oxidants hydroxyl radical (.OH), superoxide radical (O2.-), hydrogen peroxide (H2O2) and hypochlorous acid (HOCl) were studied. Their ability to inhibit iron-ion-dependent formation of .OH from H2O2 by chelating iron ions was also tested. Taurine does not react rapidly with O2.-, H2O2 or .OH, and the product of its reaction with HOCl is still sufficiently oxidizing to inactivate alpha 1-antiproteinase. Thus it seems unlikely that taurine functions as an antioxidant in vivo. Cysteic acid is also poorly reactive to the above oxidizing species. By contrast, hypotaurine is an excellent scavenger of .OH and HOCl and can interfere with iron-ion-dependent formation of .OH, although no reaction with O2.- or H2O2 could be detected within the limits of our assay techniques. Cysteamine is an excellent scavenger of .OH and HOCl; it also reacts with H2O2, but no reaction with O2.- could be measured within the limits of our assay techniques. It is concluded that cysteamine and hypotaurine are far more likely to act as antioxidants in vivo than is taurine, provided that they are present in sufficient concentration at sites of oxidant generation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruoma O. I., Halliwell B. The iron-binding and hydroxyl radical scavenging action of anti-inflammatory drugs. Xenobiotica. 1988 Apr;18(4):459–470. doi: 10.3109/00498258809041682. [DOI] [PubMed] [Google Scholar]
- Aruoma O. I., Wasil M., Halliwell B., Hoey B. M., Butler J. The scavenging of oxidants by sulphasalazine and its metabolites. A possible contribution to their anti-inflammatory effects? Biochem Pharmacol. 1987 Nov 1;36(21):3739–3742. doi: 10.1016/0006-2952(87)90028-1. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Butler J., Koppenol W. H., Margoliash E. Kinetics and mechanism of the reduction of ferricytochrome c by the superoxide anion. J Biol Chem. 1982 Sep 25;257(18):10747–10750. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Forni L. G., Willson R. L. Thiyl free radicals and the oxidation of ferrocytochrome c. Direct observation of coupled hydrogen-atom- and electron-transfer reactions. Biochem J. 1986 Dec 15;240(3):905–907. doi: 10.1042/bj2400905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M. Ferrous-salt-promoted damage to deoxyribose and benzoate. The increased effectiveness of hydroxyl-radical scavengers in the presence of EDTA. Biochem J. 1987 May 1;243(3):709–714. doi: 10.1042/bj2430709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M. Reactivity of hydroxyl and hydroxyl-like radicals discriminated by release of thiobarbituric acid-reactive material from deoxy sugars, nucleosides and benzoate. Biochem J. 1984 Dec 15;224(3):761–767. doi: 10.1042/bj2240761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Grootveld M., Gutteridge J. M. Methods for the measurement of hydroxyl radicals in biomedical systems: deoxyribose degradation and aromatic hydroxylation. Methods Biochem Anal. 1988;33:59–90. doi: 10.1002/9780470110546.ch2. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M., Aruoma O. I. The deoxyribose method: a simple "test-tube" assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987 Aug 15;165(1):215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Use of desferrioxamine as a 'probe' for iron-dependent formation of hydroxyl radicals. Evidence for a direct reaction between desferal and the superoxide radical. Biochem Pharmacol. 1985 Jan 15;34(2):229–233. doi: 10.1016/0006-2952(85)90129-7. [DOI] [PubMed] [Google Scholar]
- Hiller K. O., Hodd P. L., Willson R. L. Antiinflammatory drugs: protection of a bacterial virus as an in vitro biological measure of free radical activity. Chem Biol Interact. 1983 Dec;47(3):293–305. doi: 10.1016/0009-2797(83)90165-5. [DOI] [PubMed] [Google Scholar]
- Kuo C. F., Mashino T., Fridovich I. alpha, beta-Dihydroxyisovalerate dehydratase. A superoxide-sensitive enzyme. J Biol Chem. 1987 Apr 5;262(10):4724–4727. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Schurr A., Rigor B. M. The mechanism of neuronal resistance and adaptation to hypoxia. FEBS Lett. 1987 Nov 16;224(1):4–8. doi: 10.1016/0014-5793(87)80411-8. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Grisham M. B., Melton D. F., Jefferson M. M. Evidence for a role of taurine in the in vitro oxidative toxicity of neutrophils toward erythrocytes. J Biol Chem. 1985 Mar 25;260(6):3321–3329. [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Wasil M., Halliwell B., Hutchison D. C., Baum H. The antioxidant action of human extracellular fluids. Effect of human serum and its protein components on the inactivation of alpha 1-antiproteinase by hypochlorous acid and by hydrogen peroxide. Biochem J. 1987 Apr 1;243(1):219–223. doi: 10.1042/bj2430219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasil M., Halliwell B., Moorhouse C. P., Hutchison D. C., Baum H. Biologically-significant scavenging of the myeloperoxidase-derived oxidant hypochlorous acid by some anti-inflammatory drugs. Biochem Pharmacol. 1987 Nov 15;36(22):3847–3850. doi: 10.1016/0006-2952(87)90448-5. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Lampert M. B., Test S. T. Long-lived oxidants generated by human neutrophils: characterization and bioactivity. Science. 1983 Nov 11;222(4624):625–628. doi: 10.1126/science.6635660. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Oxygen, ischemia and inflammation. Acta Physiol Scand Suppl. 1986;548:9–37. [PubMed] [Google Scholar]
- Wright C. E., Tallan H. H., Lin Y. Y., Gaull G. E. Taurine: biological update. Annu Rev Biochem. 1986;55:427–453. doi: 10.1146/annurev.bi.55.070186.002235. [DOI] [PubMed] [Google Scholar]


