Abstract
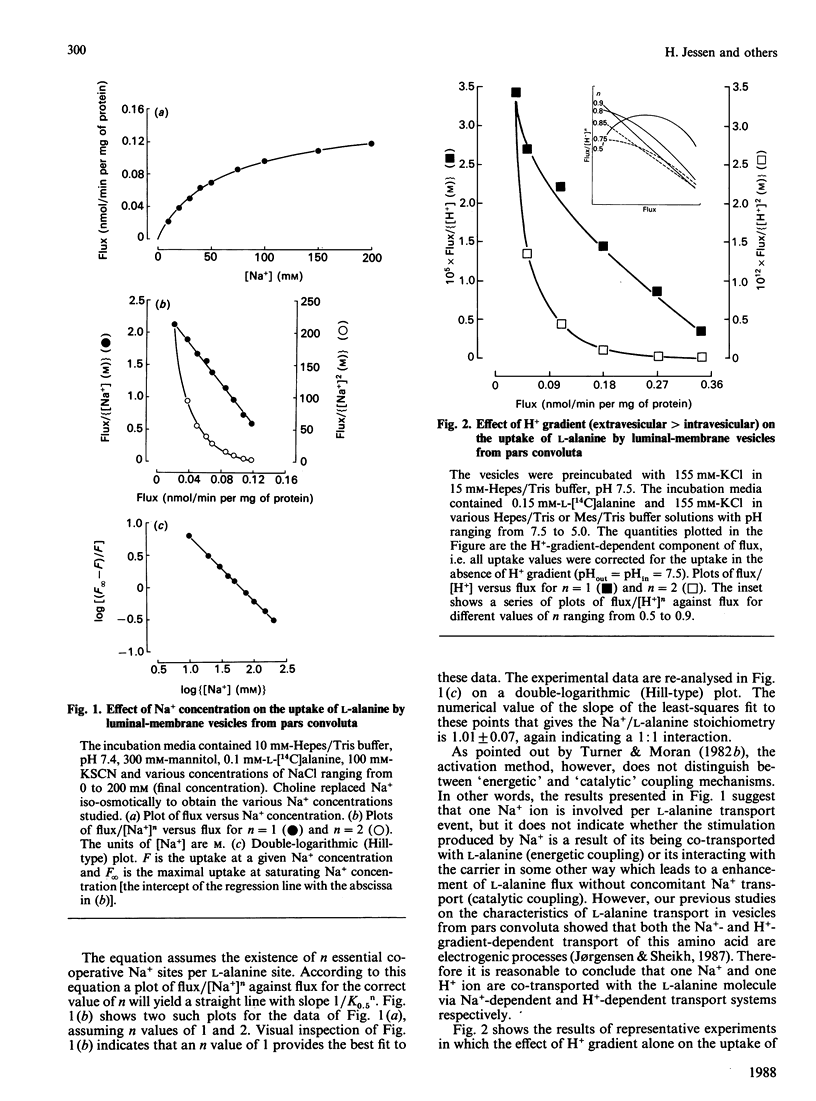
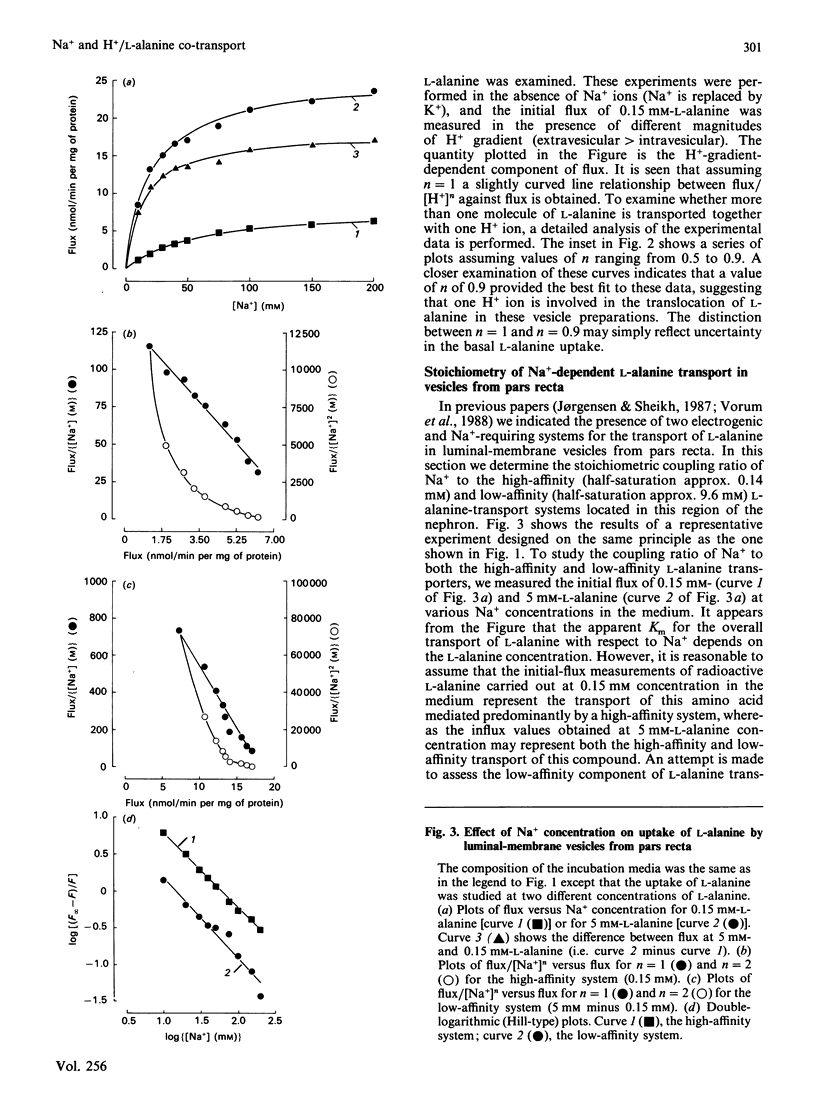
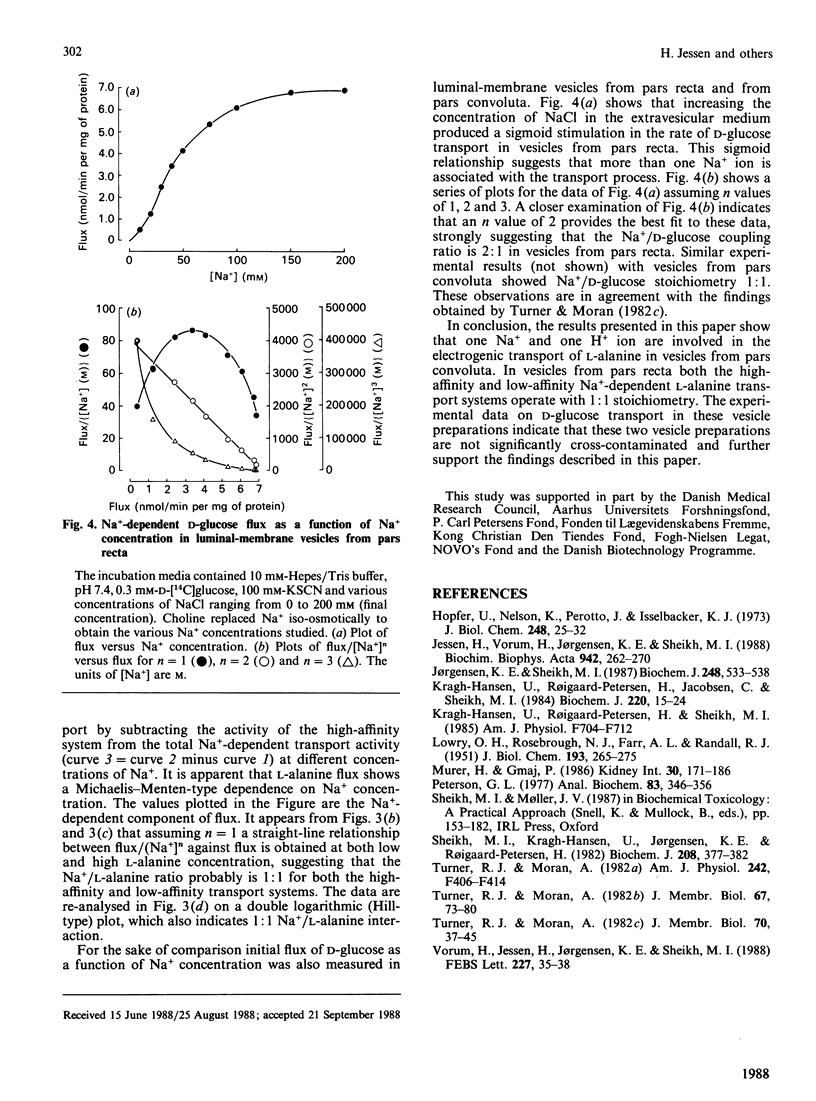
The stoichiometric properties of Na+- and H+-dependent L-alanine transporters recently identified in luminal-membrane vesicles prepared from proximal convoluted tubules (pars convoluta) and proximal straight tubules (pars recta) of rabbit kidney were studied. We provide indirect evidence suggesting that one Na+ and one H+ ion are co-transported with the L-alanine molecule via Na+-dependent and H+-dependent transport systems located in vesicles from pars convoluta. Furthermore, our experimental data suggest that both the high-affinity and the low-affinity Na+-dependent L-alanine transport systems of pars recta vesicles operate with a 1:1 stoichiometry.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Jessen H., Vorum H., Jørgensen K. E., Sheikh M. I. Characteristics of D-alanine transport by luminal membrane vesicles from pars convoluta and pars recta of rabbit proximal tubule. Biochim Biophys Acta. 1988 Jul 21;942(2):262–270. doi: 10.1016/0005-2736(88)90028-4. [DOI] [PubMed] [Google Scholar]
- Jørgensen K. E., Sheikh M. I. Renal transport of neutral amino acids. Cation-dependent uptake of L-alanine by luminal-membrane vesicles. Biochem J. 1987 Dec 1;248(2):533–538. doi: 10.1042/bj2480533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh-Hansen U., Røigaard-Petersen H., Jacobsen C., Sheikh M. I. Renal transport of neutral amino acids. Tubular localization of Na+-dependent phenylalanine- and glucose-transport systems. Biochem J. 1984 May 15;220(1):15–24. doi: 10.1042/bj2200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh-Hansen U., Røigaard-Petersen H., Sheikh M. I. Segmental localization of the rabbit renal proximal tubular Na+-H+ exchange system. Am J Physiol. 1985 Nov;249(5 Pt 2):F704–F712. doi: 10.1152/ajprenal.1985.249.5.F704. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murer H., Gmaj P. Transport studies in plasma membrane vesicles isolated from renal cortex. Kidney Int. 1986 Aug;30(2):171–186. doi: 10.1038/ki.1986.169. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Sheikh M. I., Kragh-Hansen U., Jørgensen K. E., Røigaard-Petersen H. An efficient method for the isolation and separation of basolateral-membrane and luminal-membrane vesicles from rabbit kidney cortex. Biochem J. 1982 Nov 15;208(2):377–382. doi: 10.1042/bj2080377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. J., Moran A. Further studies of proximal tubular brush border membrane D-glucose transport heterogeneity. J Membr Biol. 1982;70(1):37–45. doi: 10.1007/BF01871587. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Moran A. Heterogeneity of sodium-dependent D-glucose transport sites along the proximal tubule: evidence from vesicle studies. Am J Physiol. 1982 Apr;242(4):F406–F414. doi: 10.1152/ajprenal.1982.242.4.F406. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Moran A. Stoichiometric studies of the renal outer cortical brush border membrane D-glucose transporter. J Membr Biol. 1982;67(1):73–80. doi: 10.1007/BF01868649. [DOI] [PubMed] [Google Scholar]
- Vorum H., Jessen H., Jørgensen K. E., Sheikh M. I. Mechanism of transport of L-alanine by luminal-membrane vesicles from pars recta of rabbit proximal tubule. FEBS Lett. 1988 Jan 18;227(1):35–38. doi: 10.1016/0014-5793(88)81408-x. [DOI] [PubMed] [Google Scholar]



