Abstract
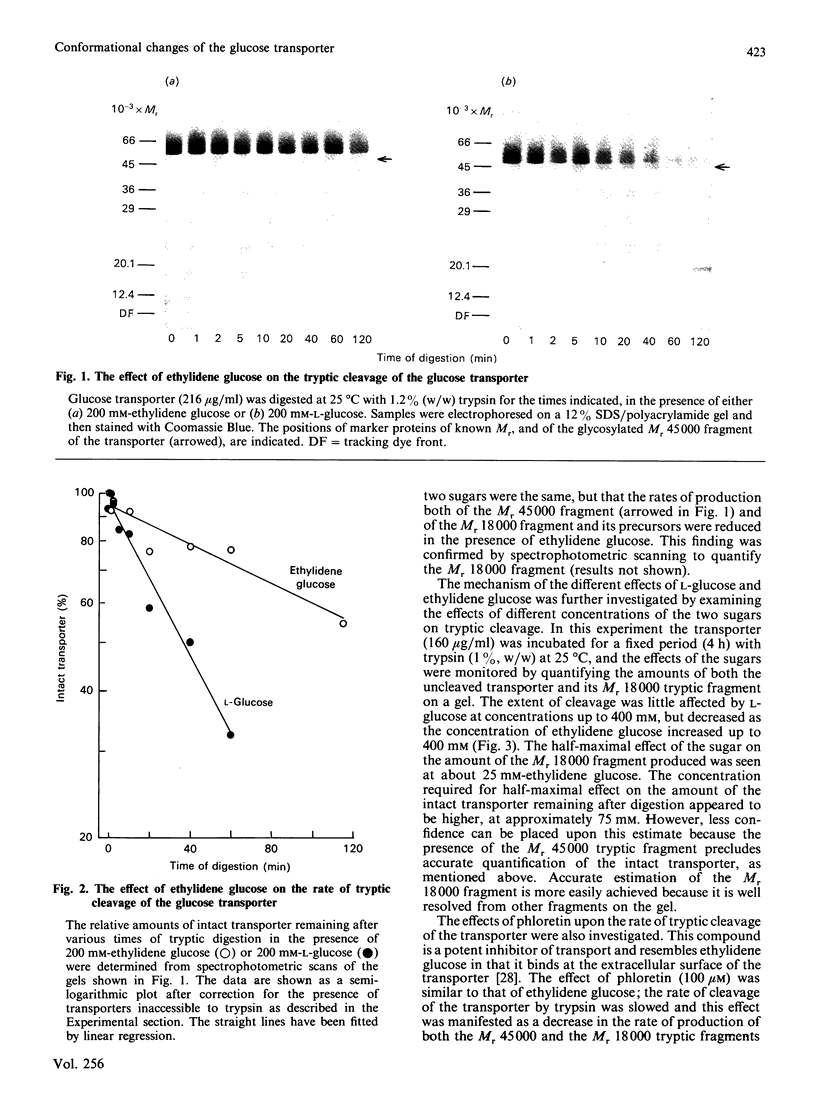
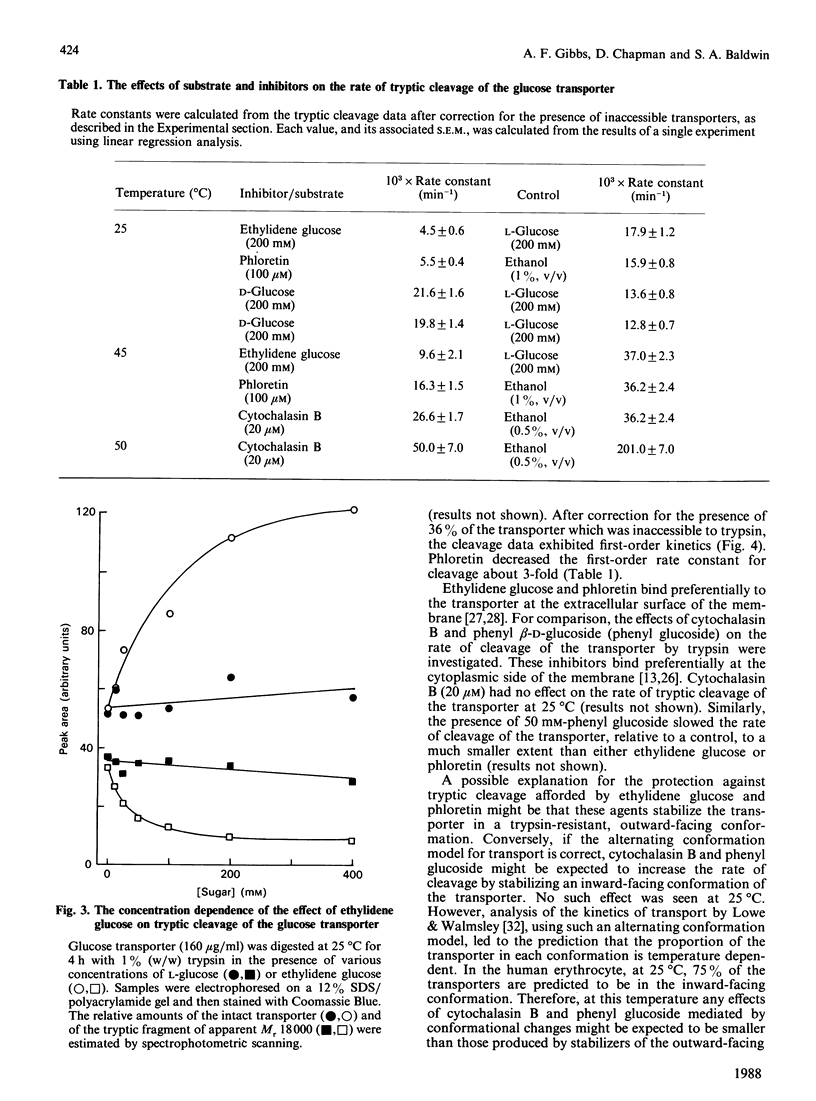
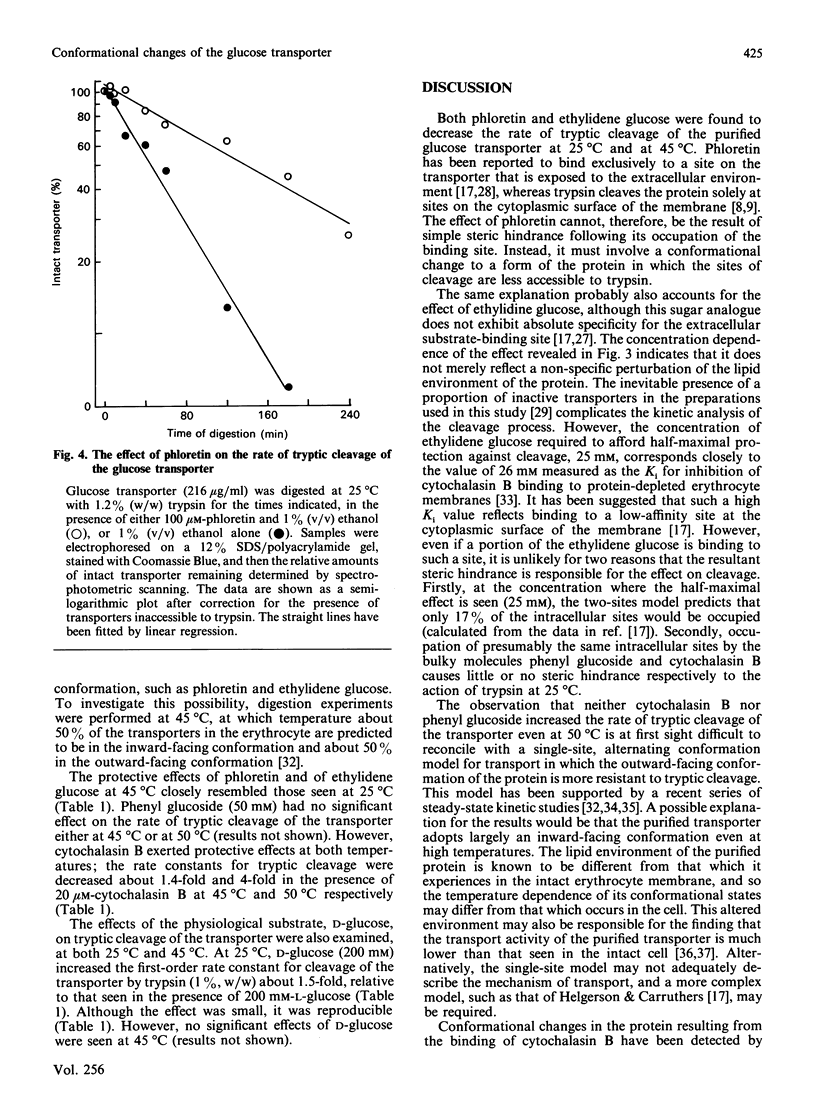
Tryptic digestion has been used to investigate the conformational changes associated with substrate translocation by the human erythrocyte glucose transporter. The effects of substrates and inhibitors of transport on the rates of tryptic cleavage at the cytoplasmic surface of the membrane have confirmed previous observations that this protein can adopt at least two conformations. In the presence of phloretin or 4,6-O-ethylidene-D-glucose, the rate of cleavage is slowed. Because these inhibitors bind preferentially at the extracellular surface of the transporter, their effects must result from a conformational change rather than from steric hindrance. A conformational change must also be responsible for the effect of the physiological substrate D-glucose, which is to increase the rate of cleavage. The regions of the protein involved in the conformational changes include both of the large cytoplasmic regions that are cleaved by trypsin: these are the central hydrophilic region of the sequence (residues 213-269) and the hydrophilic C-terminal region (residues 457-492).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez J., Lee D. C., Baldwin S. A., Chapman D. Fourier transform infrared spectroscopic study of the structure and conformational changes of the human erythrocyte glucose transporter. J Biol Chem. 1987 Mar 15;262(8):3502–3509. [PubMed] [Google Scholar]
- Appleman J. R., Lienhard G. E. Rapid kinetics of the glucose transporter from human erythrocytes. Detection and measurement of a half-turnover of the purified transporter. J Biol Chem. 1985 Apr 25;260(8):4575–4578. [PubMed] [Google Scholar]
- BOWYER F., WIDDAS W. F. The action of inhibitors on the facilitated hexose transfer system in erythrocytes. J Physiol. 1958 Apr 30;141(2):219–232. doi: 10.1113/jphysiol.1958.sp005969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker G. F., Widdas W. F. The asymmetry of the facilitated transfer system for hexoses in human red cells and the simple kinetics of a two component model. J Physiol. 1973 May;231(1):143–165. doi: 10.1113/jphysiol.1973.sp010225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin J. M., Gorga J. C., Lienhard G. E. The monosaccharide transporter of the human erythrocyte. Transport activity upon reconstitution. J Biol Chem. 1981 Apr 25;256(8):3685–3689. [PubMed] [Google Scholar]
- Baldwin S. A., Baldwin J. M., Lienhard G. E. Monosaccharide transporter of the human erythrocyte. Characterization of an improved preparation. Biochemistry. 1982 Aug 3;21(16):3836–3842. doi: 10.1021/bi00259a018. [DOI] [PubMed] [Google Scholar]
- Barnett J. E., Holman G. D., Chalkley R. A., Munday K. A. Evidence for two asymmetric conformational states in the human erythrocyte sugar-transport system. Biochem J. 1975 Mar;145(3):417–429. doi: 10.1042/bj1450417a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batt E. R., Abbott R. E., Schachter D. Impermeant maleimides. Identification of an exofacial component of the human erythrocyte hexose transport mechanism. J Biol Chem. 1976 Nov 25;251(22):7184–7190. [PubMed] [Google Scholar]
- Bloch R. Human erythrocyte sugar transport. Identification of the essential residues of the sugar carrier by specific modification. J Biol Chem. 1974 Mar 25;249(6):1814–1822. [PubMed] [Google Scholar]
- Cairns M. T., Alvarez J., Panico M., Gibbs A. F., Morris H. R., Chapman D., Baldwin S. A. Investigation of the structure and function of the human erythrocyte glucose transporter by proteolytic dissection. Biochim Biophys Acta. 1987 Dec 11;905(2):295–310. doi: 10.1016/0005-2736(87)90458-5. [DOI] [PubMed] [Google Scholar]
- Cairns M. T., Elliot D. A., Scudder P. R., Baldwin S. A. Proteolytic and chemical dissection of the human erythrocyte glucose transporter. Biochem J. 1984 Jul 1;221(1):179–188. doi: 10.1042/bj2210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers A. ATP regulation of the human red cell sugar transporter. J Biol Chem. 1986 Aug 25;261(24):11028–11037. [PubMed] [Google Scholar]
- Carruthers A. Anomalous asymmetric kinetics of human red cell hexose transfer: role of cytosolic adenosine 5'-triphosphate. Biochemistry. 1986 Jun 17;25(12):3592–3602. doi: 10.1021/bi00360a018. [DOI] [PubMed] [Google Scholar]
- Chin J. J., Jung E. K., Chen V., Jung C. Y. Structural basis of human erythrocyte glucose transporter function in proteoliposome vesicles: circular dichroism measurements. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4113–4116. doi: 10.1073/pnas.84.12.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. J., Jung E. K., Jung C. Y. Structural basis of human erythrocyte glucose transporter function in reconstituted vesicles. J Biol Chem. 1986 Jun 5;261(16):7101–7104. [PubMed] [Google Scholar]
- DAWSON A. C., WIDDAS W. F. INHIBITION OF THE GLUCOSE PERMEABILITY OF HUMAN ERYTHROCYTES BY N-ETHYL MALEIMIDE. J Physiol. 1963 Oct;168:644–659. doi: 10.1113/jphysiol.1963.sp007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A., Meeran K., Cairns M. T., Baldwin S. A. Peptide-specific antibodies as probes of the orientation of the glucose transporter in the human erythrocyte membrane. J Biol Chem. 1987 Jul 5;262(19):9347–9352. [PubMed] [Google Scholar]
- Devés R., Krupka R. M. Cytochalasin B and the kinetics of inhibition of biological transport: a case of asymmetric binding to the glucose carrier. Biochim Biophys Acta. 1978 Jul 4;510(2):339–348. doi: 10.1016/0005-2736(78)90034-2. [DOI] [PubMed] [Google Scholar]
- Deziel M. R., Jung C. Y., Rothstein A. The topology of the major band 4.5 protein component of the human erythrocyte membrane: characterization of reactive cysteine residues. Biochim Biophys Acta. 1985 Sep 25;819(1):83–92. doi: 10.1016/0005-2736(85)90198-1. [DOI] [PubMed] [Google Scholar]
- Deziel M. R., Rothstein A. Proteolytic cleavages of cytochalasin B binding components of band 4.5 proteins of the human red blood cell membrane. Biochim Biophys Acta. 1984 Sep 19;776(1):10–20. doi: 10.1016/0005-2736(84)90245-1. [DOI] [PubMed] [Google Scholar]
- Gorga F. R., Lienhard G. E. Changes in the intrinsic fluorescence of the human erythrocyte monosaccharide transporter upon ligand binding. Biochemistry. 1982 Apr 13;21(8):1905–1908. doi: 10.1021/bi00537a031. [DOI] [PubMed] [Google Scholar]
- Gorga F. R., Lienhard G. E. Equilibria and kinetics of ligand binding to the human erythrocyte glucose transporter. Evidence for an alternating conformation model for transport. Biochemistry. 1981 Sep 1;20(18):5108–5113. doi: 10.1021/bi00521a003. [DOI] [PubMed] [Google Scholar]
- Helgerson A. L., Carruthers A. Equilibrium ligand binding to the human erythrocyte sugar transporter. Evidence for two sugar-binding sites per carrier. J Biol Chem. 1987 Apr 25;262(12):5464–5475. [PubMed] [Google Scholar]
- Holman G. D., Rees W. D. Photolabelling of the hexose transporter at external and internal sites: fragmentation patterns and evidence for a conformational change. Biochim Biophys Acta. 1987 Mar 12;897(3):395–405. doi: 10.1016/0005-2736(87)90437-8. [DOI] [PubMed] [Google Scholar]
- Jung E. K., Chin J. J., Jung C. Y. Structural basis of human erythrocyte glucose transporter function in reconstituted system. Hydrogen exchange. J Biol Chem. 1986 Jul 15;261(20):9155–9160. [PubMed] [Google Scholar]
- Jung E. K., Chin J. J., Jung C. Y. Structural basis of human erythrocyte glucose transporter function in reconstituted system. Hydrogen exchange. J Biol Chem. 1986 Jul 15;261(20):9155–9160. [PubMed] [Google Scholar]
- Karim A. R., Rees W. D., Holman G. D. Binding of cytochalasin B to trypsin and thermolysin fragments of the human erythrocyte hexose transporter. Biochim Biophys Acta. 1987 Sep 3;902(3):402–405. doi: 10.1016/0005-2736(87)90208-2. [DOI] [PubMed] [Google Scholar]
- Krupka R. M. Asymmetrical binding of phloretin to the glucose transport system of human erythrocytes. J Membr Biol. 1985;83(1-2):71–80. doi: 10.1007/BF01868739. [DOI] [PubMed] [Google Scholar]
- Krupka R. M. Evidence for a carrier conformational change associated with sugar transport in erythrocytes. Biochemistry. 1971 Mar 30;10(7):1143–1148. doi: 10.1021/bi00783a007. [DOI] [PubMed] [Google Scholar]
- Kurokawa T., Tillotson L. G., Chen C. C., Isselbacher K. J. Solubilization and separation of the human erythrocyte D-glucose transporter covalently and noncovalently photoaffinity-labeled with [3H]cytochalasin B. Proc Natl Acad Sci U S A. 1986 Jan;83(2):479–482. doi: 10.1073/pnas.83.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Crabb J. H., Ransome K. J. Endoglycosidase f cleaves the oligosaccharides from the glucose transporter of the human erythrocyte. Biochim Biophys Acta. 1984 Jan 25;769(2):404–410. doi: 10.1016/0005-2736(84)90324-9. [DOI] [PubMed] [Google Scholar]
- Lowe A. G., Walmsley A. R. The kinetics of glucose transport in human red blood cells. Biochim Biophys Acta. 1986 May 28;857(2):146–154. doi: 10.1016/0005-2736(86)90342-1. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Pawagi A. B., Deber C. M. D-glucose binding increases secondary structure of human erythrocyte monosaccharide transport protein. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1087–1091. doi: 10.1016/0006-291x(87)91548-8. [DOI] [PubMed] [Google Scholar]
- Rampal A. L., Jung C. Y. Substrate-induced conformational change of human erythrocyte glucose transporter: inactivation by alkylating reagents. Biochim Biophys Acta. 1987 Jan 26;896(2):287–294. doi: 10.1016/0005-2736(87)90189-1. [DOI] [PubMed] [Google Scholar]
- Roberts S. J., Tanner M. J., Denton R. M. Properties of N-maleoylmethionine sulphone, a novel impermeant maleimide, and its use in the selective labelling of the erythrocyte glucose-transport system. Biochem J. 1982 Jul 1;205(1):139–145. doi: 10.1042/bj2050139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver G. A. Inhibition of parallel flux and augmentation of counter flux shown by transport models not involving a mobile carrier. J Theor Biol. 1966 Feb;10(2):301–306. doi: 10.1016/0022-5193(66)90128-7. [DOI] [PubMed] [Google Scholar]
- Wheeler T. J., Hinkle P. C. Kinetic properties of the reconstituted glucose transporter from human erythrocytes. J Biol Chem. 1981 Sep 10;256(17):8907–8914. [PubMed] [Google Scholar]
- Wheeler T. J. Kinetics of glucose transport in human erythrocytes: zero-trans efflux and infinite-trans efflux at 0 degree C. Biochim Biophys Acta. 1986 Nov 17;862(2):387–398. doi: 10.1016/0005-2736(86)90242-7. [DOI] [PubMed] [Google Scholar]
- Wheeler T. J., Whelan J. D. Infinite-cis kinetics support the carrier model for erythrocyte glucose transport. Biochemistry. 1988 Mar 8;27(5):1441–1450. doi: 10.1021/bi00405a008. [DOI] [PubMed] [Google Scholar]
- Zoccoli M. A., Baldwin S. A., Lienhard G. E. The monosaccharide transport system of the human erythrocyte. Solubilization and characterization on the basis of cytochalasin B binding. J Biol Chem. 1978 Oct 10;253(19):6923–6930. [PubMed] [Google Scholar]



