Abstract
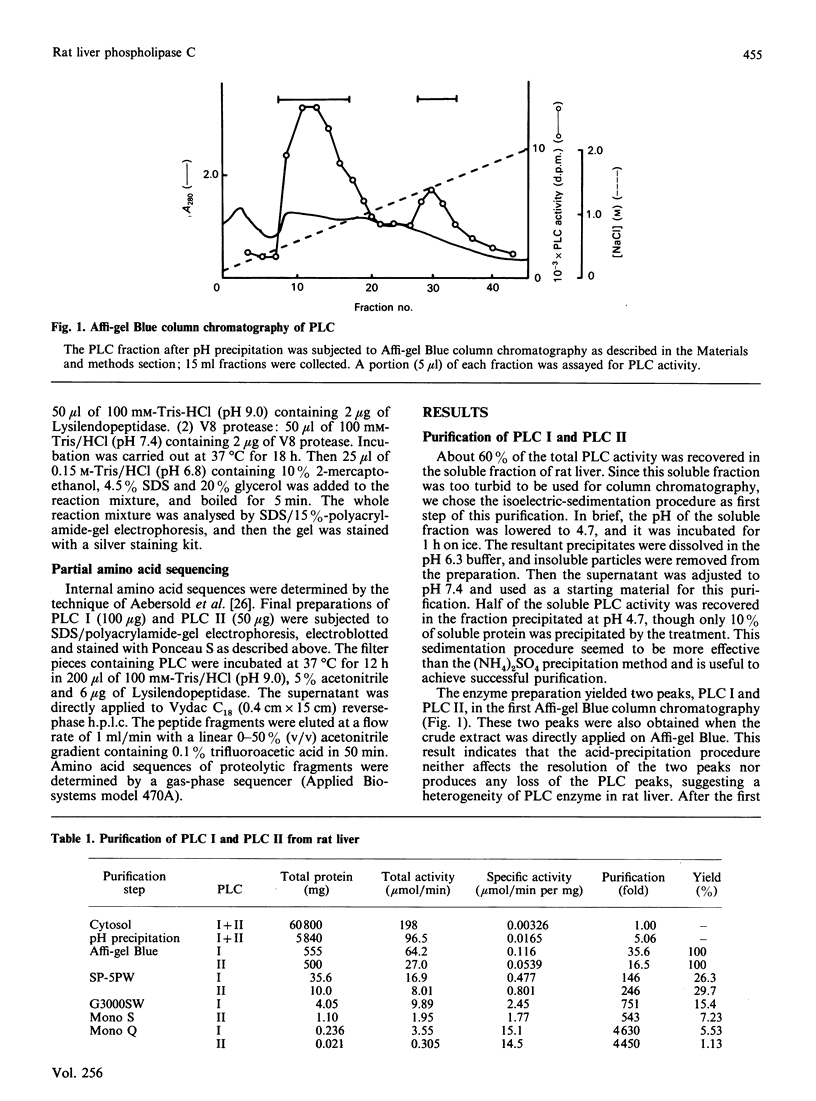
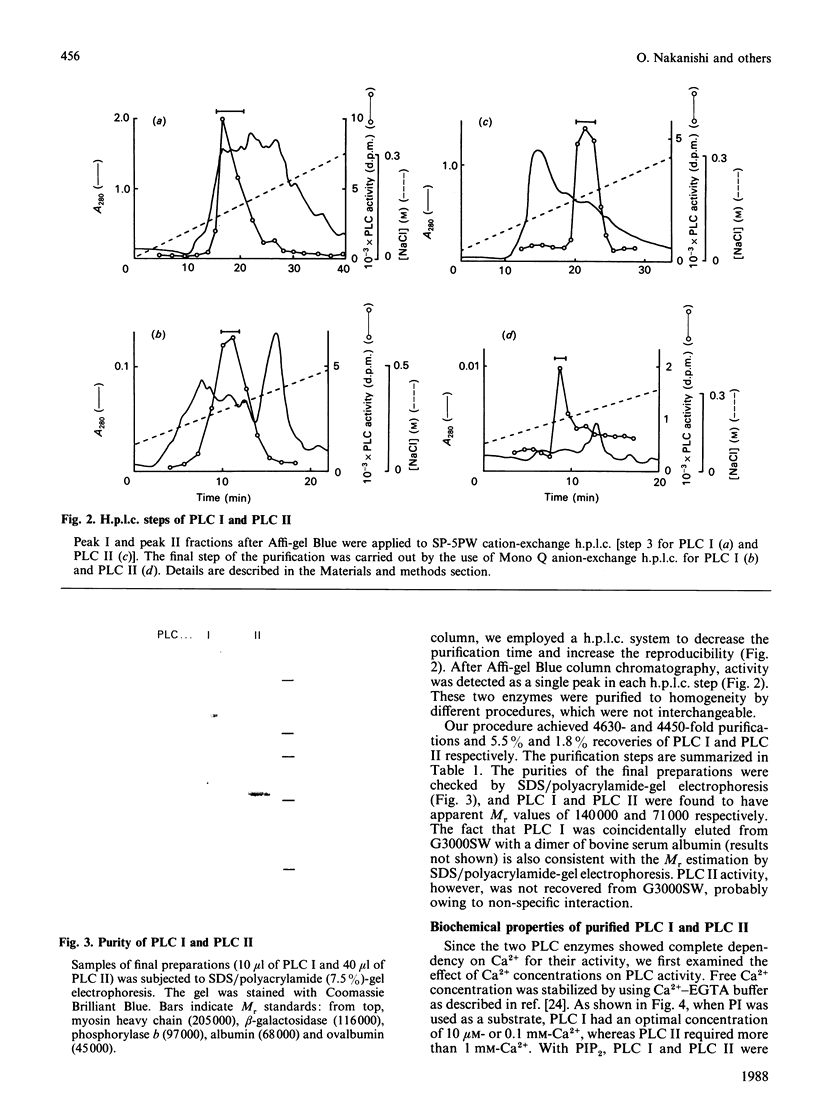
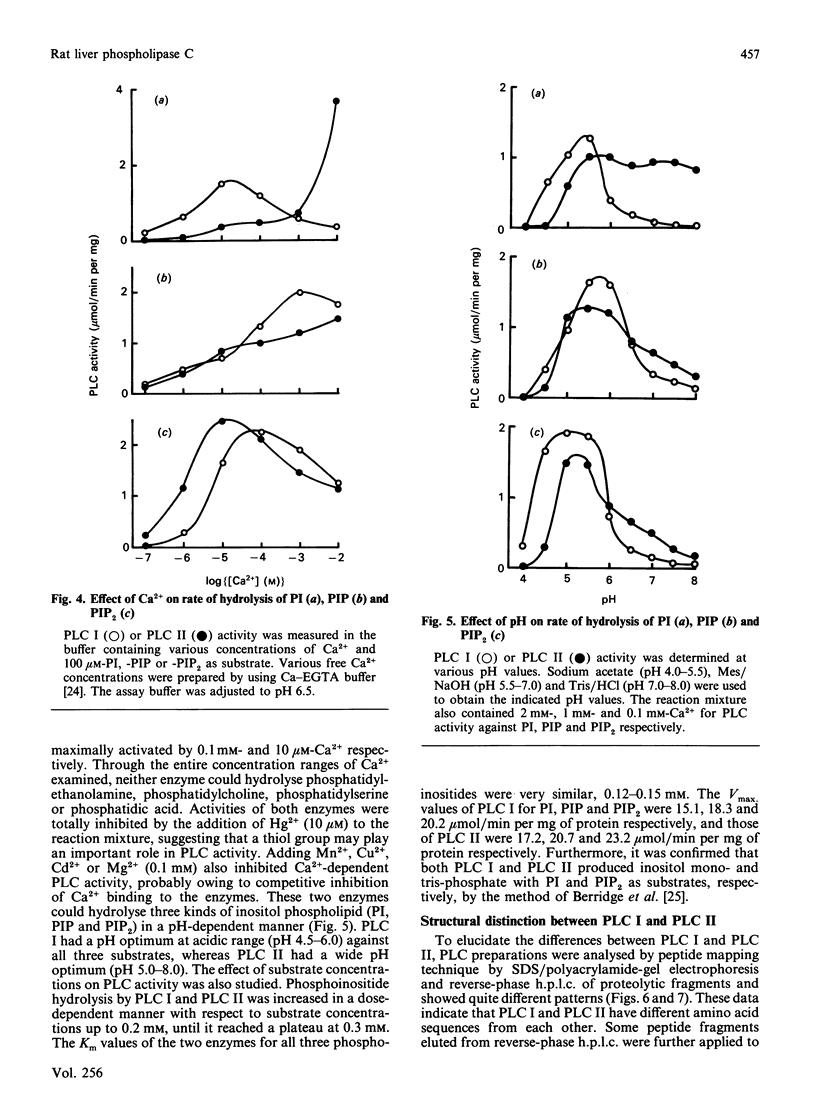
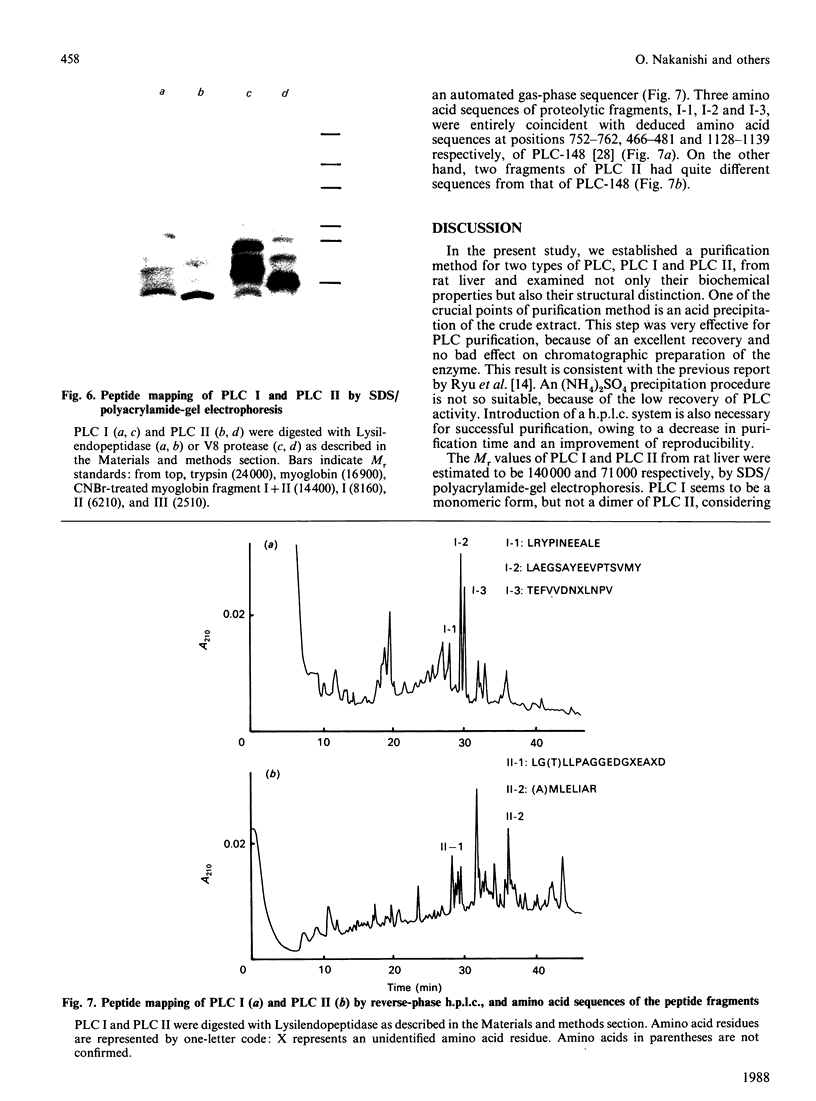
Two kinds of phosphoinositide-specific phospholipase C (PLC) were purified from rat liver by acid precipitation and several steps of column chromatography. About 50% of the activity could be precipitated when the pH of the liver homogenate was lowered to pH 4.7. The redissolved precipitate yielded two peaks, PLC I and PLC II, in an Affi-gel Blue column, and each was further purified to homogeneity by three sequential h.p.l.c. steps, which were different for the two enzymes. The purified PLC I and PLC II had estimated Mr values of 140,000 and 71,000 respectively on SDS/polyacrylamide-gel electrophoresis. Both enzymes hydrolysed phosphatidylinositol (PI), phosphatidylinositol 4-phosphate (PIP) and phosphatidylinositol 4,5-bisphosphate (PIP2) in a Ca2+- and pH-dependent manner. PLC I was most active at 10 microM- and 0.1 mM-Ca2+ for hydrolysis of PI and PIP2 respectively, whereas PLC II showed the highest activity at 5 mM- and 10 microM-Ca2+ for that of PI and PIP2 respectively. The optimal pH of the two enzymes also differed with substrates or Ca2+ concentration, in the range pH 5.0-6.0. Hydrolysis of phosphoinositides by these enzymes was completely inhibited by Hg2+ and was affected by other bivalent cations. From data obtained by peptide mapping and partial amino acid sequencing, it was clarified that PLC I and PLC II had distinct structures. Moreover, partial amino acid sequences of three proteolytic fragments of PLC I completely coincided with those of PLC-148 [Stahl, Ferenz, Kelleher, Kriz & Knopf (1988) Nature (London) 332, 269-272].
Full text
PDF


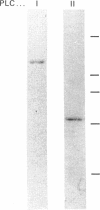
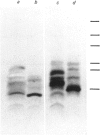
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno Y., Nakashima S., Nozawa Y. Partial purification of phosphoinositide phospholipase C from human platelet cytosol; characterization of its three forms. Biochem Biophys Res Commun. 1986 Apr 29;136(2):713–721. doi: 10.1016/0006-291x(86)90498-5. [DOI] [PubMed] [Google Scholar]
- Bennett C. F., Crooke S. T. Purification and characterization of a phosphoinositide-specific phospholipase C from guinea pig uterus. Phosphorylation by protein kinase C in vivo. J Biol Chem. 1987 Oct 5;262(28):13789–13797. [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Brandt S. J., Dougherty R. W., Lapetina E. G., Niedel J. E. Pertussis toxin inhibits chemotactic peptide-stimulated generation of inositol phosphates and lysosomal enzyme secretion in human leukemic (HL-60) cells. Proc Natl Acad Sci U S A. 1985 May;82(10):3277–3280. doi: 10.1073/pnas.82.10.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau L. Y., Tai H. H. Resolution into two different forms and study of the properties of phosphatidylinositol-specific phospholipase C from human platelet cytosol. Biochim Biophys Acta. 1982 Nov 12;713(2):344–351. [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Hakata H., Kambayashi J., Kosaki G. Purification and characterization of phosphatidylinositol-specific phospholipase C from bovine platelets. J Biochem. 1982 Sep;92(3):929–935. doi: 10.1093/oxfordjournals.jbchem.a134008. [DOI] [PubMed] [Google Scholar]
- Hirasawa K., Irvine R. F., Dawson R. M. Heterogeneity of the calcium-dependent phosphatidylinositol phosphodiesterase in rat brain. Biochem J. 1982 Aug 1;205(2):437–442. doi: 10.1042/bj2050437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann S. L., Majerus P. W. Identification and properties of two distinct phosphatidylinositol-specific phospholipase C enzymes from sheep seminal vesicular glands. J Biol Chem. 1982 Jun 10;257(11):6461–6469. [PubMed] [Google Scholar]
- Homma Y., Imaki J., Nakanishi O., Takenawa T. Isolation and characterization of two different forms of inositol phospholipid-specific phospholipase C from rat brain. J Biol Chem. 1988 May 15;263(14):6592–6598. [PubMed] [Google Scholar]
- Katan M., Parker P. J. Purification of phosphoinositide-specific phospholipase C from a particulate fraction of bovine brain. Eur J Biochem. 1987 Oct 15;168(2):413–418. doi: 10.1111/j.1432-1033.1987.tb13435.x. [DOI] [PubMed] [Google Scholar]
- Kikuchi A., Kozawa O., Kaibuchi K., Katada T., Ui M., Takai Y. Direct evidence for involvement of a guanine nucleotide-binding protein in chemotactic peptide-stimulated formation of inositol bisphosphate and trisphosphate in differentiated human leukemic (HL-60) cells. Reconstitution with Gi or Go of the plasma membranes ADP-ribosylated by pertussis toxin. J Biol Chem. 1986 Sep 5;261(25):11558–11562. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee K. Y., Ryu S. H., Suh P. G., Choi W. C., Rhee S. G. Phospholipase C associated with particulate fractions of bovine brain. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5540–5544. doi: 10.1073/pnas.84.16.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Carroll R. C., Weglicki W. B. Multiple forms of phosphoinositide-specific phospholipase C of different relative molecular masses in animal tissues. Evidence for modification of the platelet enzyme by Ca2+-dependent proteinase. Biochem J. 1984 Aug 1;221(3):813–820. doi: 10.1042/bj2210813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus P. W., Neufeld E. J., Wilson D. B. Production of phosphoinositide-derived messengers. Cell. 1984 Jul;37(3):701–703. doi: 10.1016/0092-8674(84)90405-7. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Hamaguchi M., Hanafusa H. A novel viral oncogene with structural similarity to phospholipase C. Nature. 1988 Mar 17;332(6161):272–275. doi: 10.1038/332272a0. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Receptor-mediated inhibition of adenylate cyclase and stimulation of arachidonic acid release in 3T3 fibroblasts. Selective susceptibility to islet-activating protein, pertussis toxin. J Biol Chem. 1985 Jun 25;260(12):7226–7233. [PubMed] [Google Scholar]
- Nakamura T., Ui M. Simultaneous inhibitions of inositol phospholipid breakdown, arachidonic acid release, and histamine secretion in mast cells by islet-activating protein, pertussis toxin. A possible involvement of the toxin-specific substrate in the Ca2+-mobilizing receptor-mediated biosignaling system. J Biol Chem. 1985 Mar 25;260(6):3584–3593. [PubMed] [Google Scholar]
- Nakanishi H., Nomura H., Kikkawa U., Kishimoto A., Nishizuka Y. Rat brain and liver soluble phospholipase C: resolution of two forms with different requirements for calcium. Biochem Biophys Res Commun. 1985 Oct 30;132(2):582–590. doi: 10.1016/0006-291x(85)91173-8. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Okajima F., Katada T., Ui M. Coupling of the guanine nucleotide regulatory protein to chemotactic peptide receptors in neutrophil membranes and its uncoupling by islet-activating protein, pertussis toxin. A possible role of the toxin substrate in Ca2+-mobilizing receptor-mediated signal transduction. J Biol Chem. 1985 Jun 10;260(11):6761–6768. [PubMed] [Google Scholar]
- RAAFLAUB J. Applications of metal buffers and metal indicators in biochemistry. Methods Biochem Anal. 1956;3:301–325. doi: 10.1002/9780470110195.ch10. [DOI] [PubMed] [Google Scholar]
- Rebecchi M. J., Rosen O. M. Purification of a phosphoinositide-specific phospholipase C from bovine brain. J Biol Chem. 1987 Sep 15;262(26):12526–12532. [PubMed] [Google Scholar]
- Ryu S. H., Cho K. S., Lee K. Y., Suh P. G., Rhee S. G. Purification and characterization of two immunologically distinct phosphoinositide-specific phospholipases C from bovine brain. J Biol Chem. 1987 Sep 15;262(26):12511–12518. [PubMed] [Google Scholar]
- Ryu S. H., Cho K. S., Lee K. Y., Suh P. G., Rhee S. G. Two forms of phosphatidylinositol-specific phospholipase C from bovine brain. Biochem Biophys Res Commun. 1986 Nov 26;141(1):137–144. doi: 10.1016/s0006-291x(86)80345-x. [DOI] [PubMed] [Google Scholar]
- Ryu S. H., Suh P. G., Cho K. S., Lee K. Y., Rhee S. G. Bovine brain cytosol contains three immunologically distinct forms of inositolphospholipid-specific phospholipase C. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6649–6653. doi: 10.1073/pnas.84.19.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht J. Purification of polyphosphoinositides by chromatography on immobilized neomycin. J Lipid Res. 1978 Nov;19(8):1063–1067. [PubMed] [Google Scholar]
- Stahl M. L., Ferenz C. R., Kelleher K. L., Kriz R. W., Knopf J. L. Sequence similarity of phospholipase C with the non-catalytic region of src. Nature. 1988 Mar 17;332(6161):269–272. doi: 10.1038/332269a0. [DOI] [PubMed] [Google Scholar]
- Straub R. E., Gershengorn M. C. Thyrotropin-releasing hormone and GTP activate inositol trisphosphate formation in membranes isolated from rat pituitary cells. J Biol Chem. 1986 Feb 25;261(6):2712–2717. [PubMed] [Google Scholar]
- Takenawa T., Nagai Y. Purification of phosphatidylinositol-specific phospholipase C from rat liver. J Biol Chem. 1981 Jul 10;256(13):6769–6775. [PubMed] [Google Scholar]
- Uhing R. J., Prpic V., Jiang H., Exton J. H. Hormone-stimulated polyphosphoinositide breakdown in rat liver plasma membranes. Roles of guanine nucleotides and calcium. J Biol Chem. 1986 Feb 15;261(5):2140–2146. [PubMed] [Google Scholar]
- Verghese M. W., Smith C. D., Snyderman R. Potential role for a guanine nucleotide regulatory protein in chemoattractant receptor mediated polyphosphoinositide metabolism, Ca++ mobilization and cellular responses by leukocytes. Biochem Biophys Res Commun. 1985 Mar 15;127(2):450–457. doi: 10.1016/s0006-291x(85)80181-9. [DOI] [PubMed] [Google Scholar]
- Volpi M., Naccache P. H., Molski T. F., Shefcyk J., Huang C. K., Marsh M. L., Munoz J., Becker E. L., Sha'afi R. I. Pertussis toxin inhibits fMet-Leu-Phe- but not phorbol ester-stimulated changes in rabbit neutrophils: role of G proteins in excitation response coupling. Proc Natl Acad Sci U S A. 1985 May;82(9):2708–2712. doi: 10.1073/pnas.82.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Toyoshima S., Osawa T. Partial purification and characterization of membrane-bound and cytosolic phosphatidylinositol-specific phospholipases C from murine thymocytes. J Biochem. 1986 Oct;100(4):1015–1022. doi: 10.1093/oxfordjournals.jbchem.a121780. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Bross T. E., Hofmann S. L., Majerus P. W. Hydrolysis of polyphosphoinositides by purified sheep seminal vesicle phospholipase C enzymes. J Biol Chem. 1984 Oct 10;259(19):11718–11724. [PubMed] [Google Scholar]