Abstract
Chinese hamster cells (V79) resistant to high concentrations of Cd2+ in the medium were obtained by using the procedure of Beach & Palmiter [(1981) Proc. Natl. Acad. Sci. U.S.A. 78, 2110-2114], which in mouse led to amplification of metallothionein (MT) genes and to an enrichment in cellular MT. The Cd-resistant V79 clones isolated were significantly more resistant than parental cells to oxidative stress by extracellular H2O2 or a mixture of H2O2 and superoxide anion (O2-) generated by xanthine oxidase plus acetaldehyde. On a per-cell basis, there was no difference between the two cells in their total H2O2-decomposing or O2-(-)dismutating activity. The most likely explanation is that an enrichment in MT content in the Cd-resistant cells was responsible for this effect, because of the antioxidant properties already described for this protein.
Full text
PDF
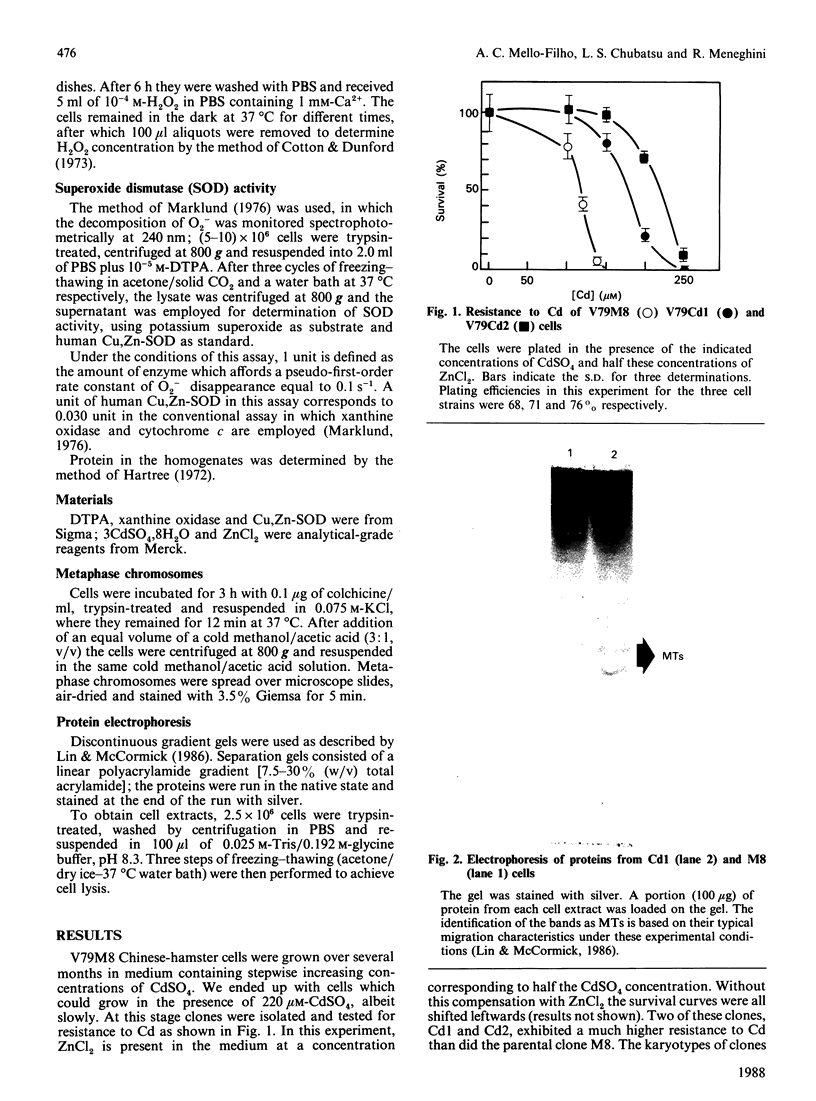
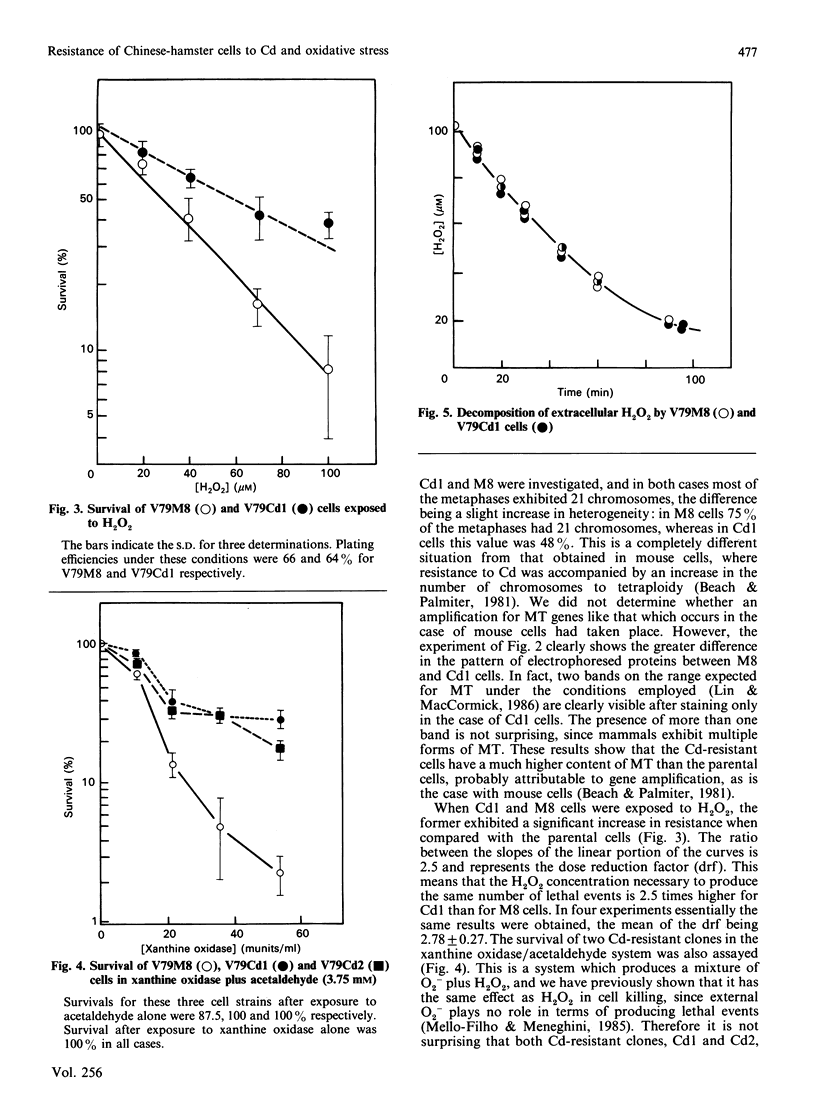
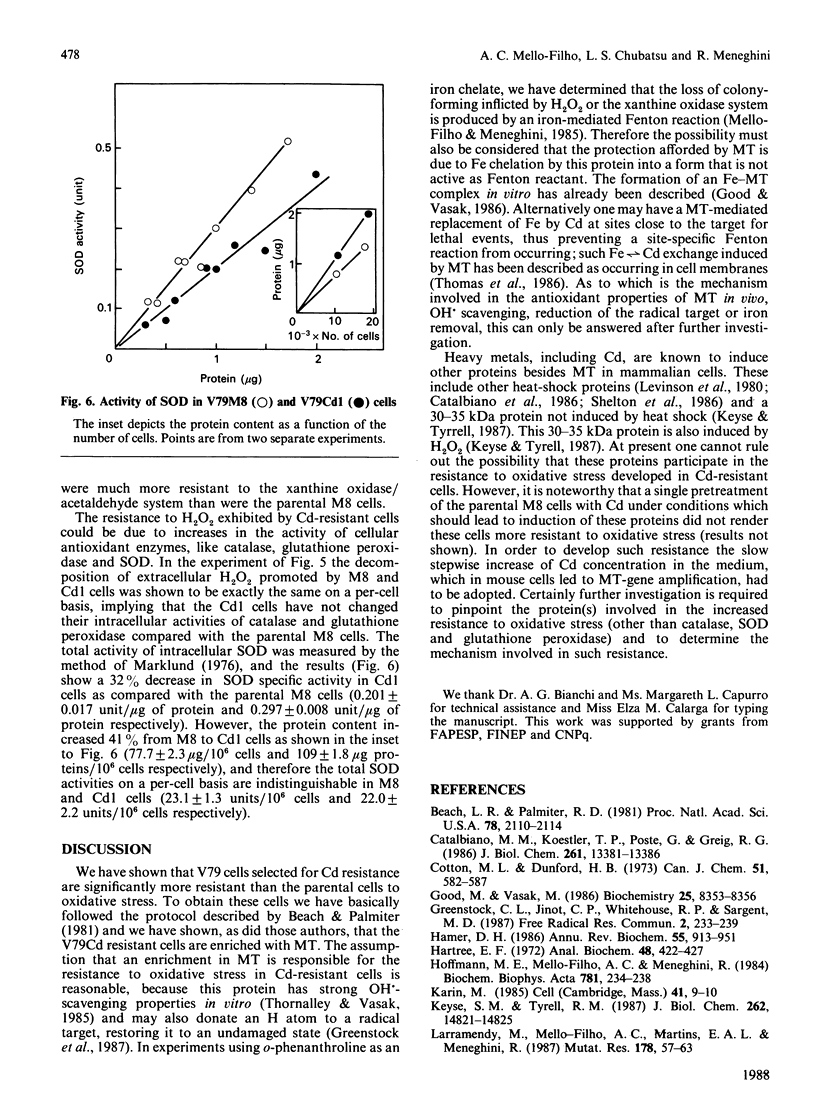

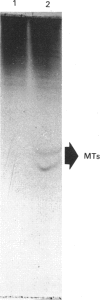
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach L. R., Palmiter R. D. Amplification of the metallothionein-I gene in cadmium-resistant mouse cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2110–2114. doi: 10.1073/pnas.78.4.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caltabiano M. M., Koestler T. P., Poste G., Greig R. G. Induction of 32- and 34-kDa stress proteins by sodium arsenite, heavy metals, and thiol-reactive agents. J Biol Chem. 1986 Oct 5;261(28):13381–13386. [PubMed] [Google Scholar]
- Good M., Vasák M. Iron(II)-substituted metallothionein: evidence for the existence of iron-thiolate clusters. Biochemistry. 1986 Dec 30;25(26):8353–8356. doi: 10.1021/bi00374a003. [DOI] [PubMed] [Google Scholar]
- Greenstock C. L., Jinot C. P., Whitehouse R. P., Sargent M. D. DNA radiation damage and its modification by metallothionein. Free Radic Res Commun. 1987;2(4-6):233–239. doi: 10.3109/10715768709065288. [DOI] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hoffmann M. E., Mello-Filho A. C., Meneghini R. Correlation between cytotoxic effect of hydrogen peroxide and the yield of DNA strand breaks in cells of different species. Biochim Biophys Acta. 1984 Apr 5;781(3):234–238. doi: 10.1016/0167-4781(84)90088-5. [DOI] [PubMed] [Google Scholar]
- Karin M. Metallothioneins: proteins in search of function. Cell. 1985 May;41(1):9–10. doi: 10.1016/0092-8674(85)90051-0. [DOI] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Both near ultraviolet radiation and the oxidizing agent hydrogen peroxide induce a 32-kDa stress protein in normal human skin fibroblasts. J Biol Chem. 1987 Oct 25;262(30):14821–14825. [PubMed] [Google Scholar]
- Larramendy M., Mello-Filho A. C., Martins E. A., Meneghini R. Iron-mediated induction of sister-chromatid exchanges by hydrogen peroxide and superoxide anion. Mutat Res. 1987 May;178(1):57–63. doi: 10.1016/0027-5107(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Levinson W., Oppermann H., Jackson J. Transition series metals and sulfhydryl reagents induce the synthesis of four proteins in eukaryotic cells. Biochim Biophys Acta. 1980;606(1):170–180. doi: 10.1016/0005-2787(80)90108-2. [DOI] [PubMed] [Google Scholar]
- Lin L. Y., McCormick C. C. Quantitation of chick tissue zinc-metallothionein by gel electrophoresis and silver stain enhancement. Comp Biochem Physiol C. 1986;85(1):75–84. doi: 10.1016/0742-8413(86)90054-x. [DOI] [PubMed] [Google Scholar]
- Marklund S. Spectrophotometric study of spontaneous disproportionation of superoxide anion radical and sensitive direct assay for superoxide dismutase. J Biol Chem. 1976 Dec 10;251(23):7504–7507. [PubMed] [Google Scholar]
- Mello Filho A. C., Hoffmann M. E., Meneghini R. Cell killing and DNA damage by hydrogen peroxide are mediated by intracellular iron. Biochem J. 1984 Feb 15;218(1):273–275. doi: 10.1042/bj2180273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello Filho A. C., Meneghini R. In vivo formation of single-strand breaks in DNA by hydrogen peroxide is mediated by the Haber-Weiss reaction. Biochim Biophys Acta. 1984 Feb 24;781(1-2):56–63. doi: 10.1016/0167-4781(84)90123-4. [DOI] [PubMed] [Google Scholar]
- Shelton K. R., Egle P. M., Todd J. M. Evidence that glutathione participates in the induction of a stress protein. Biochem Biophys Res Commun. 1986 Jan 29;134(2):492–498. doi: 10.1016/s0006-291x(86)80447-8. [DOI] [PubMed] [Google Scholar]
- Thomas J. P., Bachowski G. J., Girotti A. W. Inhibition of cell membrane lipid peroxidation by cadmium- and zinc-metallothioneins. Biochim Biophys Acta. 1986 Dec 10;884(3):448–461. doi: 10.1016/0304-4165(86)90195-9. [DOI] [PubMed] [Google Scholar]
- Thornalley P. J., Vasák M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta. 1985 Jan 21;827(1):36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- de Mello Filho A. C., Meneghini R. Protection of mammalian cells by o-phenanthroline from lethal and DNA-damaging effects produced by active oxygen species. Biochim Biophys Acta. 1985 Oct 30;847(1):82–89. doi: 10.1016/0167-4889(85)90156-9. [DOI] [PubMed] [Google Scholar]