Abstract
Protein phosphatase activity specific for Tyr(P) (phosphotyrosine) residues (PTP-phosphatase) was found in the cytosol and particulate fractions of human placenta. In the particulate fraction, half of the PTP-phosphatase activity could be extracted with 1% Triton X-100. The PTP-phosphatase remaining in the Triton-insoluble residue was solubilized with 0.6 M-KCl plus 1% CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]propane-1-sulphonate) and was purified 1850-fold by adsorption to DEAE-Sepharose, affinity chromatography on Zn2+-iminodiacetate-agarose, phosphocellulose adsorption, Fractogel filtration and Mono Q chromatography. The cytoskeleton-associated PTP-phosphatase was distinguished from acid, alkaline and other protein Ser(P) (phosphoserine)/Thr(P) (phosphothreonine) phosphatases by its neutral pH optimum, activity in the presence of EDTA, inhibition by Zn2+, vanadate, or molybdate, and low activity with either [Ser(P)]phosphorylase a or p-nitrophenyl phosphate. The PTP-phosphate displayed a Km of 0.15 microM with [Tyr(P)]serum albumin as substrate, 10-100-fold lower than the Km for previously described protein phosphatases. The cytoskeleton-associated PTP-phosphatase catalysed the dephosphorylation of receptors for insulin, insulin-like growth factor-1 and epidermal growth factor labelled by autophosphorylation. The properties of this PTP-phosphatase suggest that it plays a role in the regulation of hormone receptors and cytoskeleton proteins by reversible phosphorylation on tyrosine residues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arino J., Woon C. W., Brautigan D. L., Miller T. B., Jr, Johnson G. L. Human liver phosphatase 2A: cDNA and amino acid sequence of two catalytic subunit isotypes. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4252–4256. doi: 10.1073/pnas.85.12.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brautigan D. L., Bornstein P., Gallis B. Phosphotyrosyl-protein phosphatase. Specific inhibition by Zn. J Biol Chem. 1981 Jul 10;256(13):6519–6522. [PubMed] [Google Scholar]
- Brown S., Levinson W., Spudich J. A. Cytoskeletal elements of chick embryo fibroblasts revealed by detergent extraction. J Supramol Struct. 1976;5(2):119–130. doi: 10.1002/jss.400050203. [DOI] [PubMed] [Google Scholar]
- Böni-Schnetzler M., Pilch P. F. Mechanism of epidermal growth factor receptor autophosphorylation and high-affinity binding. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7832–7836. doi: 10.1073/pnas.84.22.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Clari G., Brunati A. M., Moret V. Partial purification and characterization of phosphotyrosyl-protein phosphatase(s) from human erythrocyte cytosol. Biochem Biophys Res Commun. 1986 May 29;137(1):566–572. doi: 10.1016/0006-291x(86)91248-9. [DOI] [PubMed] [Google Scholar]
- Cobb M. H., Rosen O. M. The insulin receptor and tyrosine protein kinase activity. Biochim Biophys Acta. 1984;738(1-2):1–8. doi: 10.1016/0304-419x(84)90016-7. [DOI] [PubMed] [Google Scholar]
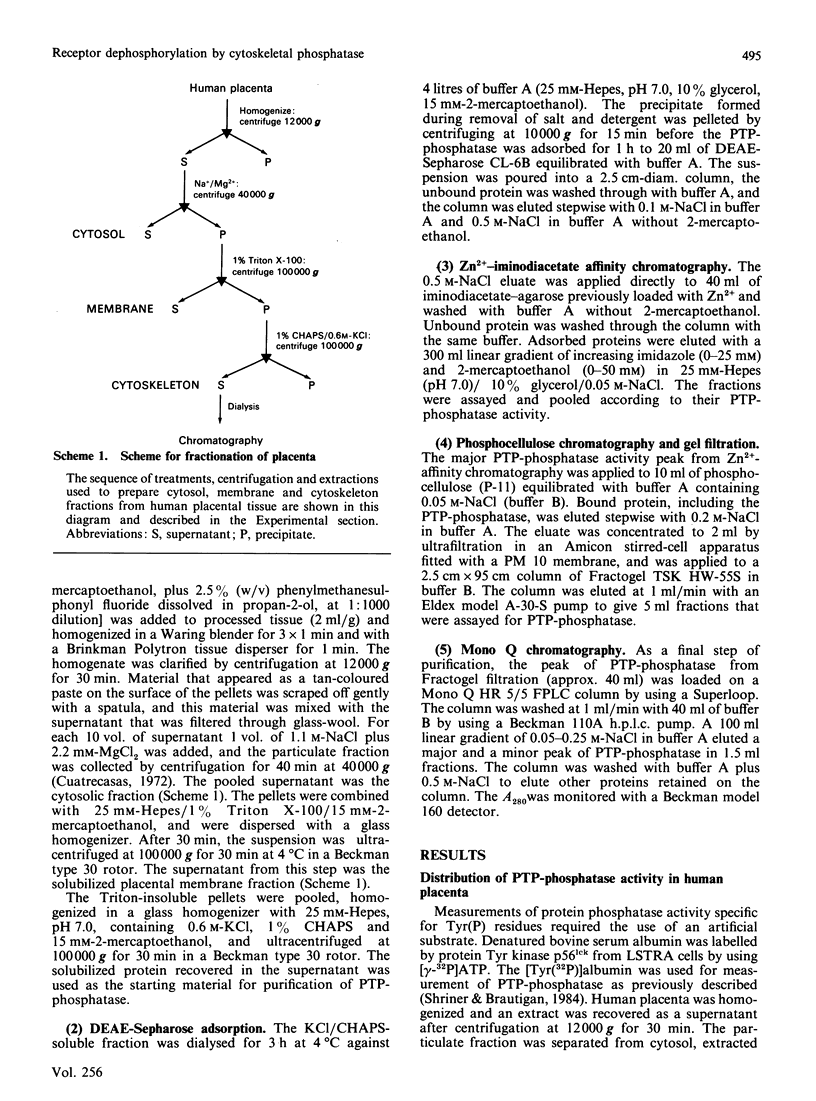
- Cuatrecasas P. Isolation of the insulin receptor of liver and fat-cell membranes (detergent-solubilized-( 125 I)insulin-polyethylene glycol precipitation-sephadex). Proc Natl Acad Sci U S A. 1972 Feb;69(2):318–322. doi: 10.1073/pnas.69.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaoli-Roach A. A., Ahmad Z., Roach P. J. Characterization of a rabbit skeletal muscle protein kinase (PC0.7) able to phosphorylate glycogen synthase and phosvitin. J Biol Chem. 1981 Sep 10;256(17):8955–8962. [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes J. G., Howard R. F., Ziemiecki A. Detection of a novel mammalian protein phosphatase with activity for phosphotyrosine. FEBS Lett. 1981 Aug 3;130(2):197–200. doi: 10.1016/0014-5793(81)81118-0. [DOI] [PubMed] [Google Scholar]
- Gallis B., Bornstein P., Brautigan D. L. Tyrosylprotein kinase and phosphatase activities in membrane vesicles from normal and Rous sarcoma virus-transformed rat cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6689–6693. doi: 10.1073/pnas.78.11.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M., Hanafusa H. Association of p60src with Triton X-100-resistant cellular structure correlates with morphological transformation. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2312–2316. doi: 10.1073/pnas.84.8.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
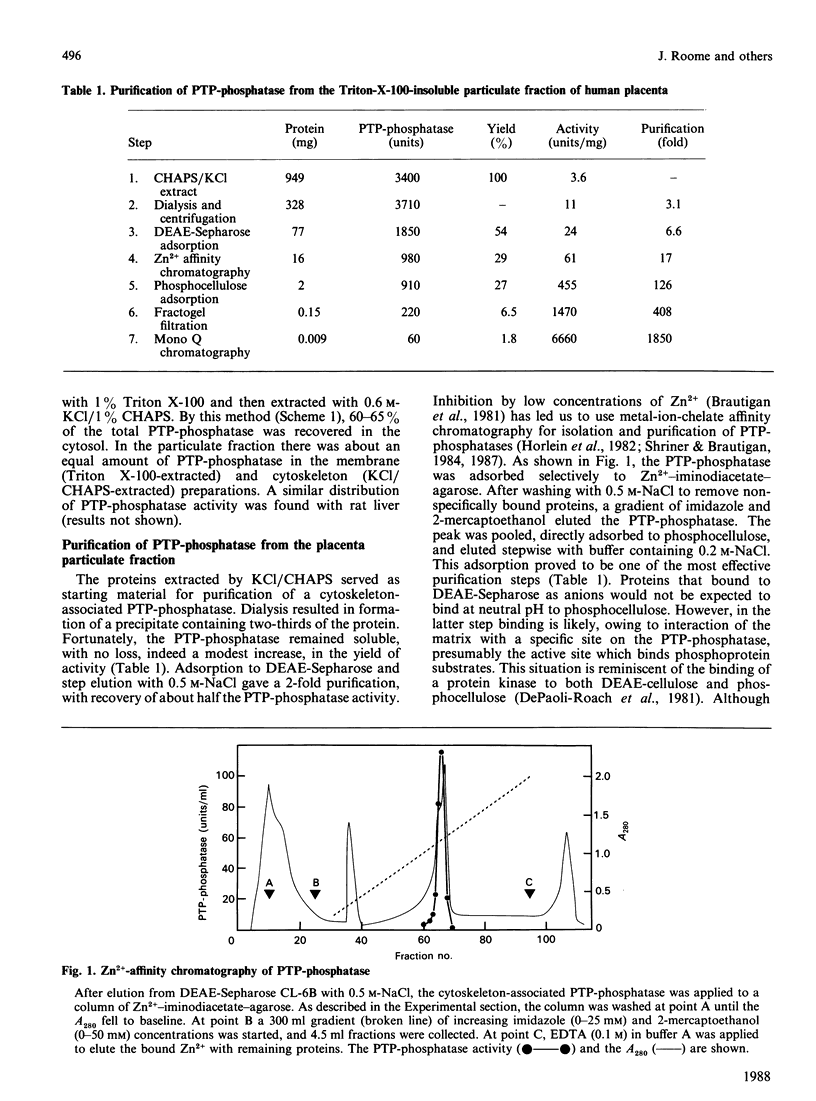
- Hörlein D., Gallis B., Brautigan D. L., Bornstein P. Partial purification and characterization of phosphotyrosyl-protein phosphatase from Ehrlich ascites tumor cells. Biochemistry. 1982 Oct 26;21(22):5577–5584. doi: 10.1021/bi00265a030. [DOI] [PubMed] [Google Scholar]
- Leis J. F., Knowles A. F., Kaplan N. O. Demonstration of separate phosphotyrosyl- and phosphoseryl- histone phosphatase activities in the plasma membranes of a human astrocytoma. Arch Biochem Biophys. 1985 Jun;239(2):320–326. doi: 10.1016/0003-9861(85)90694-0. [DOI] [PubMed] [Google Scholar]
- Maher P. A., Pasquale E. B., Wang J. Y., Singer S. J. Phosphotyrosine-containing proteins are concentrated in focal adhesions and intercellular junctions in normal cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6576–6580. doi: 10.1073/pnas.82.19.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. L., Branton P. E. Identification, purification, and characterization of phosphotyrosine-specific protein phosphatases from cultured chicken embryo fibroblasts. Mol Cell Biol. 1984 Jun;4(6):1003–1012. doi: 10.1128/mcb.4.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. The detertent-resistant cytoskeleton of tissue culture cells includes the nucleus and the microfilament bundles. Exp Cell Res. 1977 May;106(2):339–349. doi: 10.1016/0014-4827(77)90179-3. [DOI] [PubMed] [Google Scholar]
- Rotenberg S. A., Brautigan D. L. Membrane protein phosphotyrosine phosphatase in rabbit kidney. Proteolysis activates the enzyme and generates soluble catalytic fragments. Biochem J. 1987 May 1;243(3):747–754. doi: 10.1042/bj2430747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. Allosteric regulation of the epidermal growth factor receptor kinase. J Cell Biol. 1986 Dec;103(6 Pt 1):2067–2072. doi: 10.1083/jcb.103.6.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T. Tyrosine protein kinases. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:195–226. [PubMed] [Google Scholar]
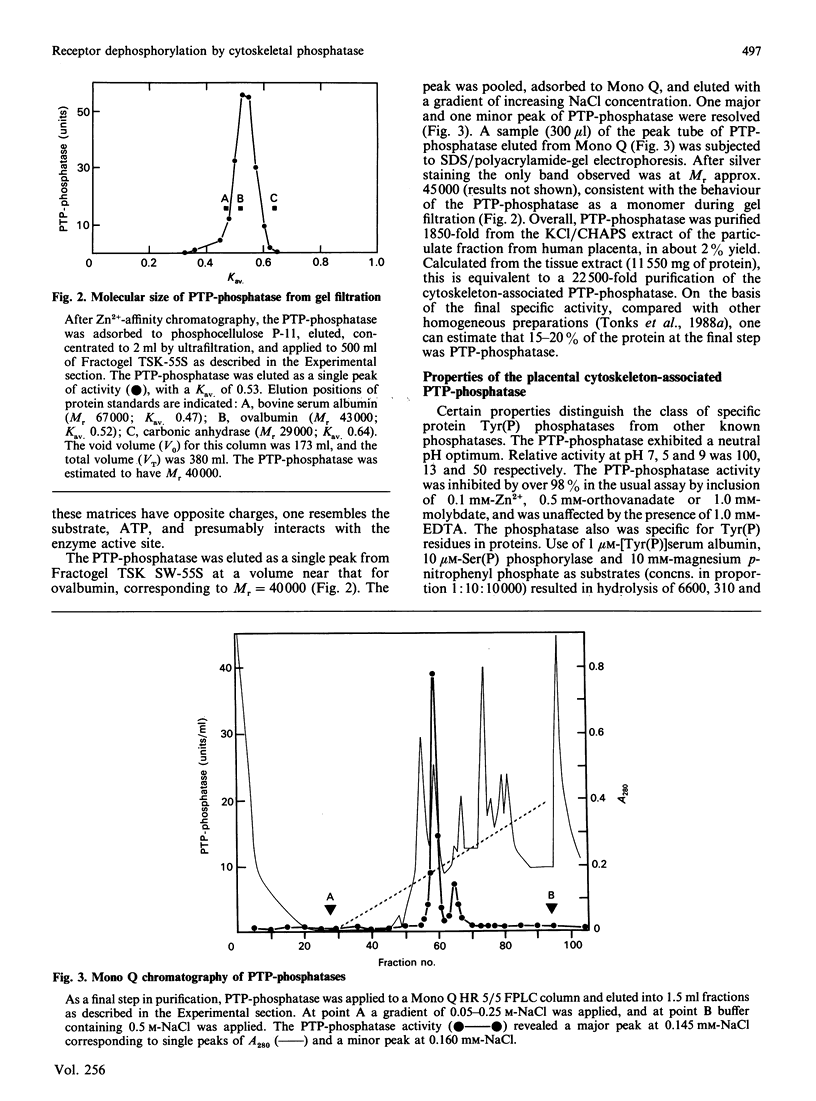
- Shriner C. L., Brautigan D. L. Cytosolic protein phosphotyrosine phosphatases from rabbit kidney. Purification of two distinct enzymes that bind to Zn2+-iminodiacetate agarose. J Biol Chem. 1984 Sep 25;259(18):11383–11390. [PubMed] [Google Scholar]
- Shriner C. L., Brautigan D. L. Metal chelate affinity chromatography of Zn2+-inhibited protein Tyr(P) phosphatases. J Biochem Biophys Methods. 1987 Aug;14(5):273–278. doi: 10.1016/0165-022x(87)90053-4. [DOI] [PubMed] [Google Scholar]
- Strout H. V., Vicario P. P., Saperstein R., Slater E. E. A protein phosphotyrosine phosphatase distinct from alkaline phosphatase with activity against the insulin receptor. Biochem Biophys Res Commun. 1988 Mar 15;151(2):633–640. doi: 10.1016/s0006-291x(88)80328-0. [DOI] [PubMed] [Google Scholar]
- Swarup G., Cohen S., Garbers D. L. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Commun. 1982 Aug;107(3):1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- Tollefsen S. E., Thompson K., Petersen D. J. Separation of the high affinity insulin-like growth factor I receptor from low affinity binding sites by affinity chromatography. J Biol Chem. 1987 Dec 5;262(34):16461–16469. [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. Characterization of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988 May 15;263(14):6731–6737. [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. Purification of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988 May 15;263(14):6722–6730. [PubMed] [Google Scholar]
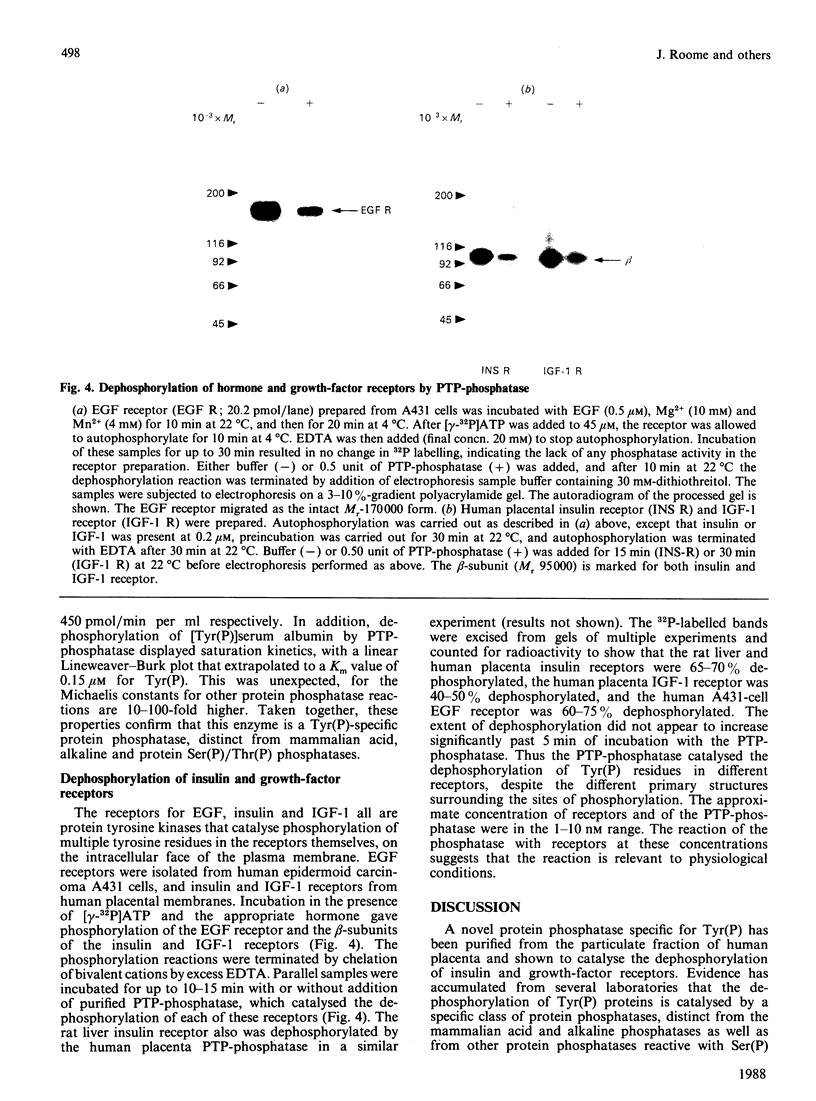
- Tornqvist H. E., Pierce M. W., Frackelton A. R., Nemenoff R. A., Avruch J. Identification of insulin receptor tyrosine residues autophosphorylated in vitro. J Biol Chem. 1987 Jul 25;262(21):10212–10219. [PubMed] [Google Scholar]
- White M. F., Shoelson S. E., Keutmann H., Kahn C. R. A cascade of tyrosine autophosphorylation in the beta-subunit activates the phosphotransferase of the insulin receptor. J Biol Chem. 1988 Feb 25;263(6):2969–2980. [PubMed] [Google Scholar]



