Abstract
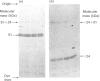
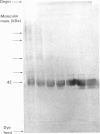
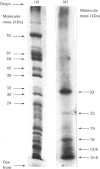
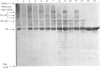
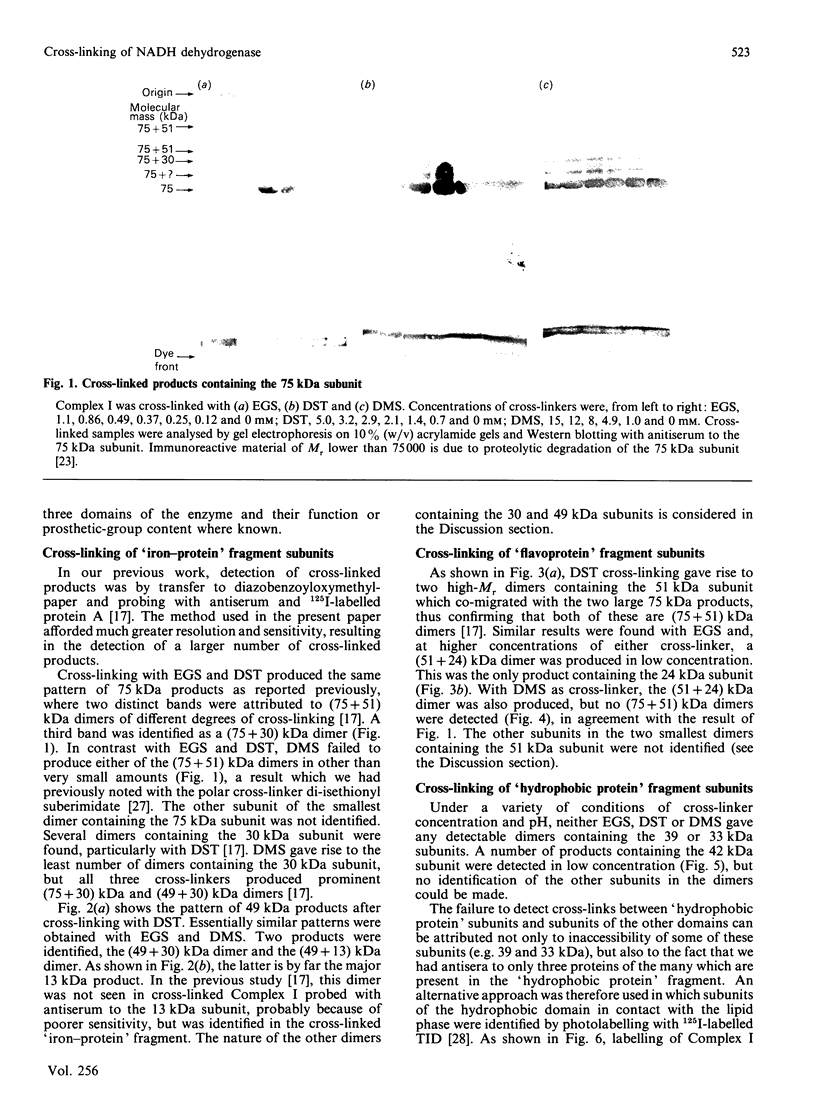
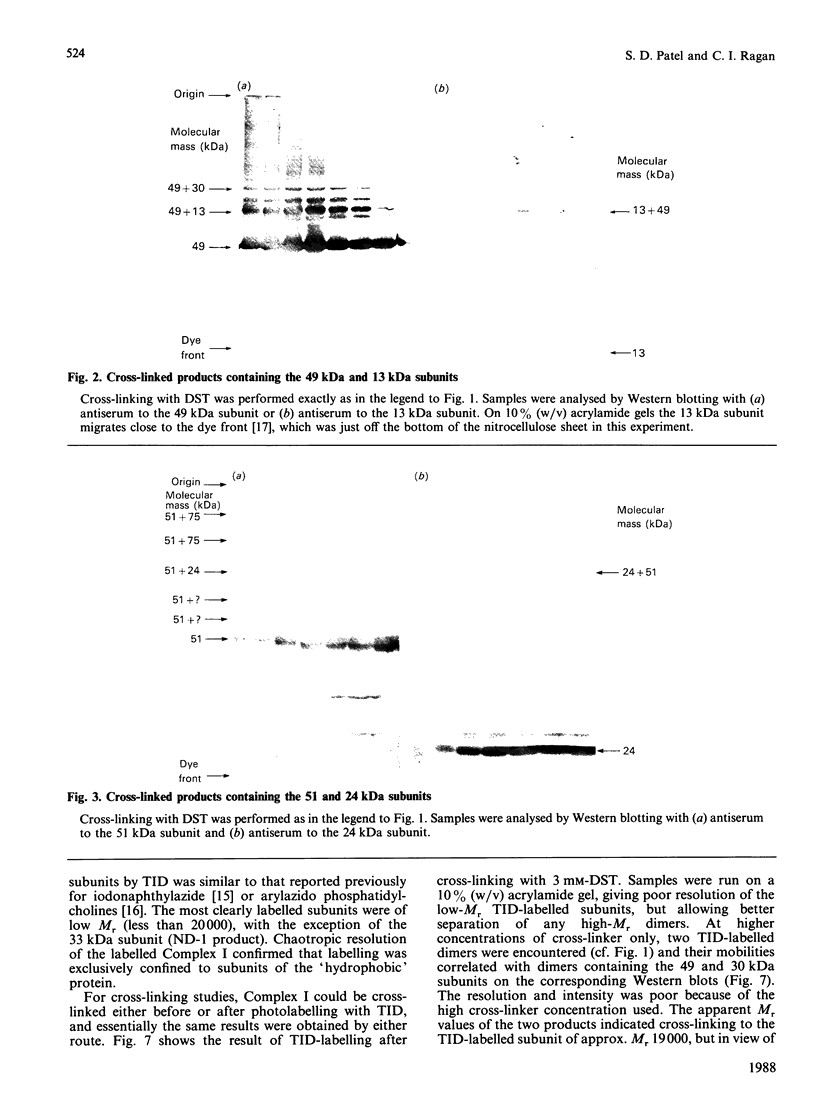
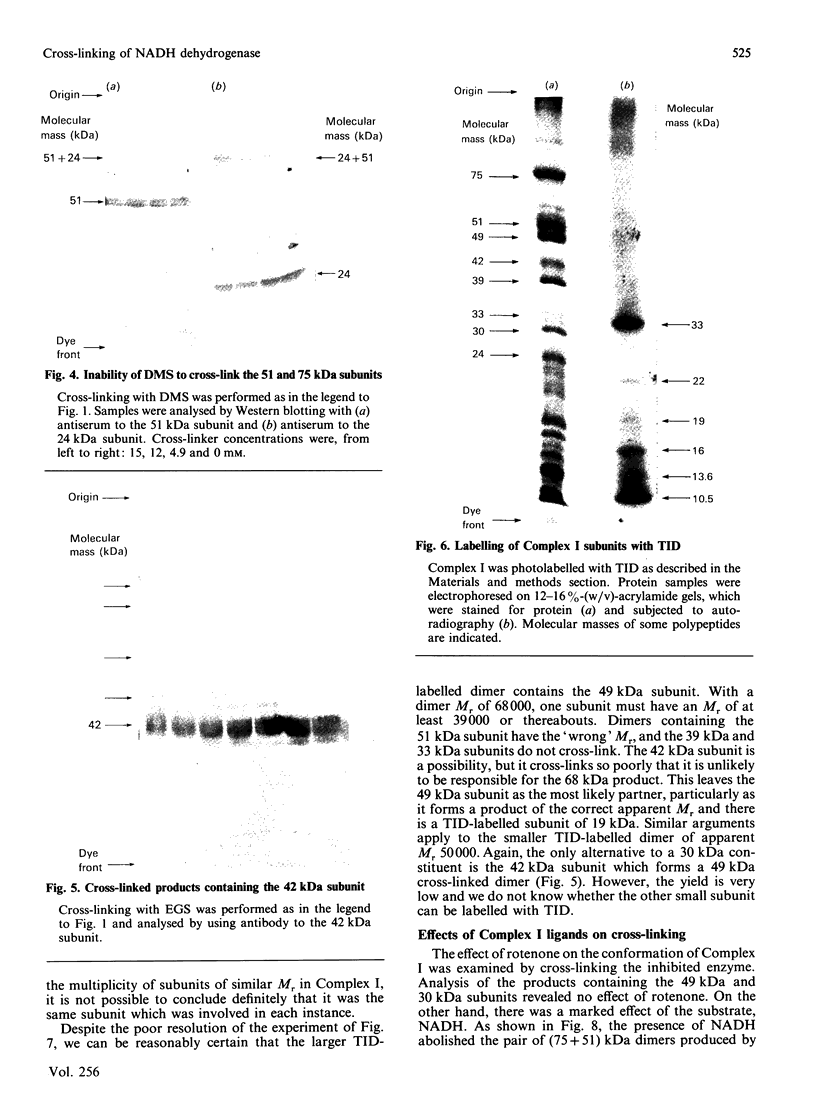
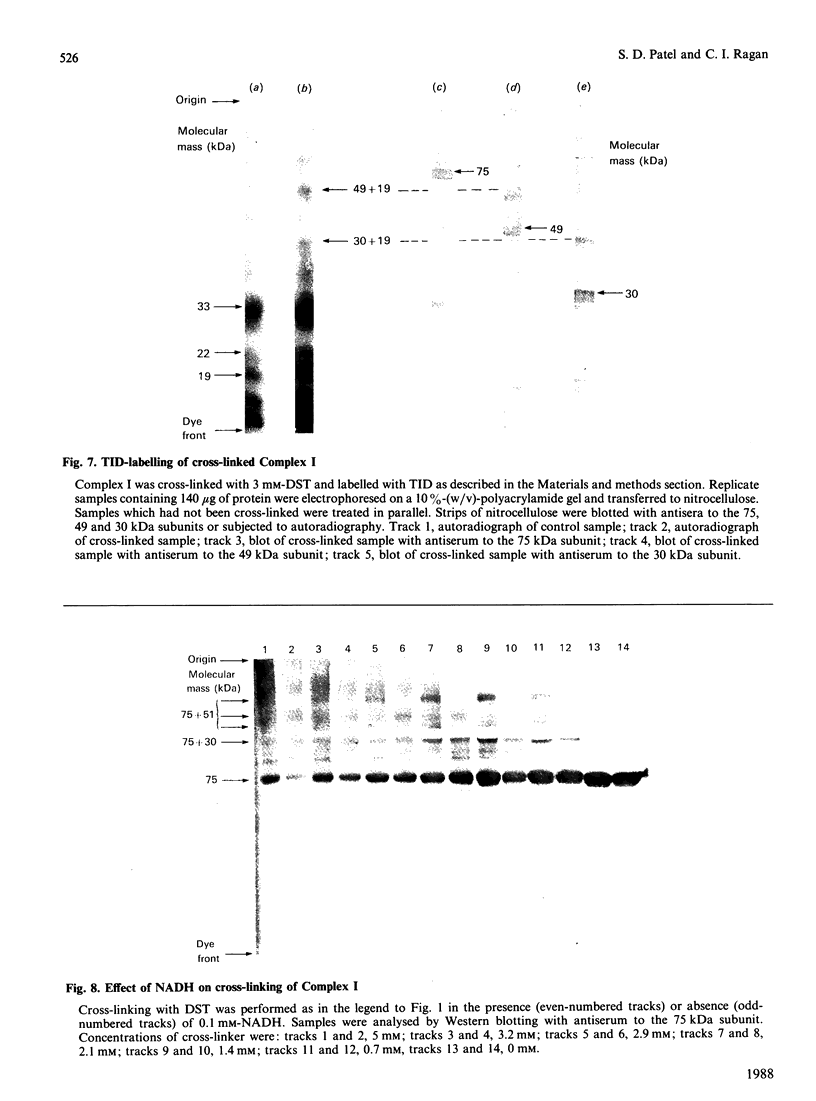
The structure of bovine heart mitochondrial NADH dehydrogenase was investigated by cross-linking constituent subunits with disuccinimidyl tartrate, (ethylene glycol)yl bis(succinimidyl succinate) and dimethyl suberimidate. Cross-linked products were identified by Western blotting with monospecific antisera to nine subunits of the enzyme. Cross-links between subunits within the flavoprotein, iron-protein and hydrophobic domains of the enzyme were identified. Cross-linking between the 75 kDa iron-protein-domain subunit and the 51 kDa flavoprotein-domain subunit was modulated by the substrate NADH. Cross-linking of subunits of the iron-protein and flavoprotein domains to constituents of the hydrophobic domain was also found. This was further substantiated by photolabelling subunits of the latter region, which were in contact with the membrane lipid, with 3-(trifluoromethyl)-3-(m-[125I]iodophenyl)diazirine. One such subunit of Mr 19,000 could be cross-linked to components of the iron-protein domain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beinert H., Albracht S. P. New insights, ideas and unanswered questions concerning iron-sulfur clusters in mitochondria. Biochim Biophys Acta. 1982 Dec 31;683(3-4):245–277. doi: 10.1016/0304-4173(82)90003-9. [DOI] [PubMed] [Google Scholar]
- Bisson R., Schiavo G. Two different forms of cytochrome c oxidase can be purified from the slime mold Dictyostelium discoideum. J Biol Chem. 1986 Apr 5;261(10):4373–4376. [PubMed] [Google Scholar]
- Boekema E. J., Van Heel M. G., Van Bruggen E. F. Three-dimensional structure of bovine NADH:ubiquinone oxidoreductase of the mitochondrial respiratory chain. Biochim Biophys Acta. 1984 May 31;787(1):19–26. doi: 10.1016/0167-4838(84)90103-1. [DOI] [PubMed] [Google Scholar]
- Brink J., Hovmöller S., Ragan C. I., Cleeter M. W., Boekema E. J., van Bruggen E. F. The structure of NADH:ubiquinone oxidoreductase from beef-heart mitochondria. Crystals containing an octameric arrangement of iron-sulphur protein fragments. Eur J Biochem. 1987 Jul 15;166(2):287–294. doi: 10.1111/j.1432-1033.1987.tb13513.x. [DOI] [PubMed] [Google Scholar]
- Brunner J., Semenza G. Selective labeling of the hydrophobic core of membranes with 3-(trifluoromethyl)-3-(m-[125I]iodophenyl)diazirine, a carbene-generating reagent. Biochemistry. 1981 Dec 8;20(25):7174–7182. doi: 10.1021/bi00528a019. [DOI] [PubMed] [Google Scholar]
- Chen S., Guillory R. J. Studies on the interaction of arylazido-beta-alanyl NAD+ with the mitochondrial NADH dehydrogenase. J Biol Chem. 1981 Aug 25;256(16):8318–8323. [PubMed] [Google Scholar]
- Chomyn A., Cleeter M. W., Ragan C. I., Riley M., Doolittle R. F., Attardi G. URF6, last unidentified reading frame of human mtDNA, codes for an NADH dehydrogenase subunit. Science. 1986 Oct 31;234(4776):614–618. doi: 10.1126/science.3764430. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Mariottini P., Cleeter M. W., Ragan C. I., Matsuno-Yagi A., Hatefi Y., Doolittle R. F., Attardi G. Six unidentified reading frames of human mitochondrial DNA encode components of the respiratory-chain NADH dehydrogenase. Nature. 1985 Apr 18;314(6012):592–597. doi: 10.1038/314592a0. [DOI] [PubMed] [Google Scholar]
- Cleeter M. W., Banister S. H., Ragan C. I. Chemical cross-linking of mitochondrial NADH dehydrogenase from bovine heart. Biochem J. 1985 Apr 15;227(2):467–474. doi: 10.1042/bj2270467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeter M. W., Ragan C. I. The polypeptide composition of the mitochondrial NADH: ubiquinone reductase complex from several mammalian species. Biochem J. 1985 Sep 15;230(3):739–746. doi: 10.1042/bj2300739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley F. G., Patel S. D., Ragan I., Attardi G. Photolabelling of a mitochondrially encoded subunit of NADH dehydrogenase with [3H]dihydrorotenone. FEBS Lett. 1987 Jul 13;219(1):108–112. doi: 10.1016/0014-5793(87)81200-0. [DOI] [PubMed] [Google Scholar]
- Earley F. G., Ragan C. I. Identification of the subunits of bovine heart mitochondrial NADH dehydrogenase that are exposed to the phospholipid bilayer by photo-labelling with 5-iodonaphth-1-yl azide. Biochem J. 1980 Nov 1;191(2):429–436. doi: 10.1042/bj1910429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley F. G., Ragan C. I. Photoaffinity labelling of mitochondrial NADH dehydrogenase with arylazidoamorphigenin, an analogue of rotenone. Biochem J. 1984 Dec 1;224(2):525–534. doi: 10.1042/bj2240525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson P. F., Minier L. N., Lasher R. S. Quantitative electrophoretic transfer of polypeptides from SDS polyacrylamide gels to nitrocellulose sheets: a method for their re-use in immunoautoradiographic detection of antigens. J Immunol Methods. 1982 Jun 11;51(2):241–249. doi: 10.1016/0022-1759(82)90263-0. [DOI] [PubMed] [Google Scholar]
- Gondal J. A., Anderson W. M. The molecular morphology of bovine heart mitochondrial NADH----ubiquinone reductase. Native disulfide-linked subunits and rotenone-induced conformational changes. J Biol Chem. 1985 Oct 15;260(23):12690–12694. [PubMed] [Google Scholar]
- Gondal J. A., Anderson W. M. The molecular morphology of bovine heart mitochondrial NADH-ubiquinone reductase. Cross-linking with dithiobis(succinimidyl propionate). J Biol Chem. 1985 May 25;260(10):5931–5935. [PubMed] [Google Scholar]
- Hatefi Y., Stempel K. E. Isolation and enzymatic properties of the mitochondrial reduced diphosphopyridine nucleotide dehydrogenase. J Biol Chem. 1969 May 10;244(9):2350–2357. [PubMed] [Google Scholar]
- Kowal A. T., Morningstar J. E., Johnson M. K., Ramsay R. R., Singer T. P. Spectroscopic characterization of the number and type of iron-sulfur clusters in NADH:ubiquinone oxidoreductase. J Biol Chem. 1986 Jul 15;261(20):9239–9245. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leonard K., Haiker H., Weiss H. Three-dimensional structure of NADH: ubiquinone reductase (complex I) from Neurospora mitochondria determined by electron microscopy of membrane crystals. J Mol Biol. 1987 Mar 20;194(2):277–286. doi: 10.1016/0022-2836(87)90375-5. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Ragan C. I., Hatefi Y. EPR studies of iron-sulfur clusters in isolated subunits and subfractions of NADH-ubiquinone oxidoreductase. J Biol Chem. 1985 Mar 10;260(5):2782–2788. [PubMed] [Google Scholar]
- Patel S. D., Cleeter M. W., Ragan C. I. Transmembrane organization of mitochondrial NADH dehydrogenase as revealed by radiochemical labelling and cross-linking. Biochem J. 1988 Dec 1;256(2):529–535. doi: 10.1042/bj2560529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragan C. I., Galante Y. M., Hatefi Y., Ohnishi T. Resolution of mitochondrial NADH dehydrogenase and isolation of two iron-sulfur proteins. Biochemistry. 1982 Feb 2;21(3):590–594. doi: 10.1021/bi00532a027. [DOI] [PubMed] [Google Scholar]
- Ragan C. I., Galante Y. M., Hatefi Y. Purification of three iron-sulfur proteins from the iron-protein fragment of mitochondrial NADH-ubiquinone oxidoreductase. Biochemistry. 1982 May 11;21(10):2518–2524. doi: 10.1021/bi00539a035. [DOI] [PubMed] [Google Scholar]
- Smith S., Ragan C. I. The organization of NADH dehydrogenase polypeptides in the inner mitochondrial membrane. Biochem J. 1980 Feb 1;185(2):315–326. doi: 10.1042/bj1850315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor V. J., Ragan C. I. The induction of mitochondrial L-3-glycerophosphate dehydrogenase by thyroid hormone. Biochim Biophys Acta. 1986 Aug 13;851(1):49–56. doi: 10.1016/0005-2728(86)90247-1. [DOI] [PubMed] [Google Scholar]