Abstract
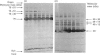
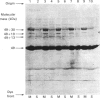
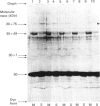
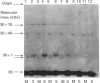
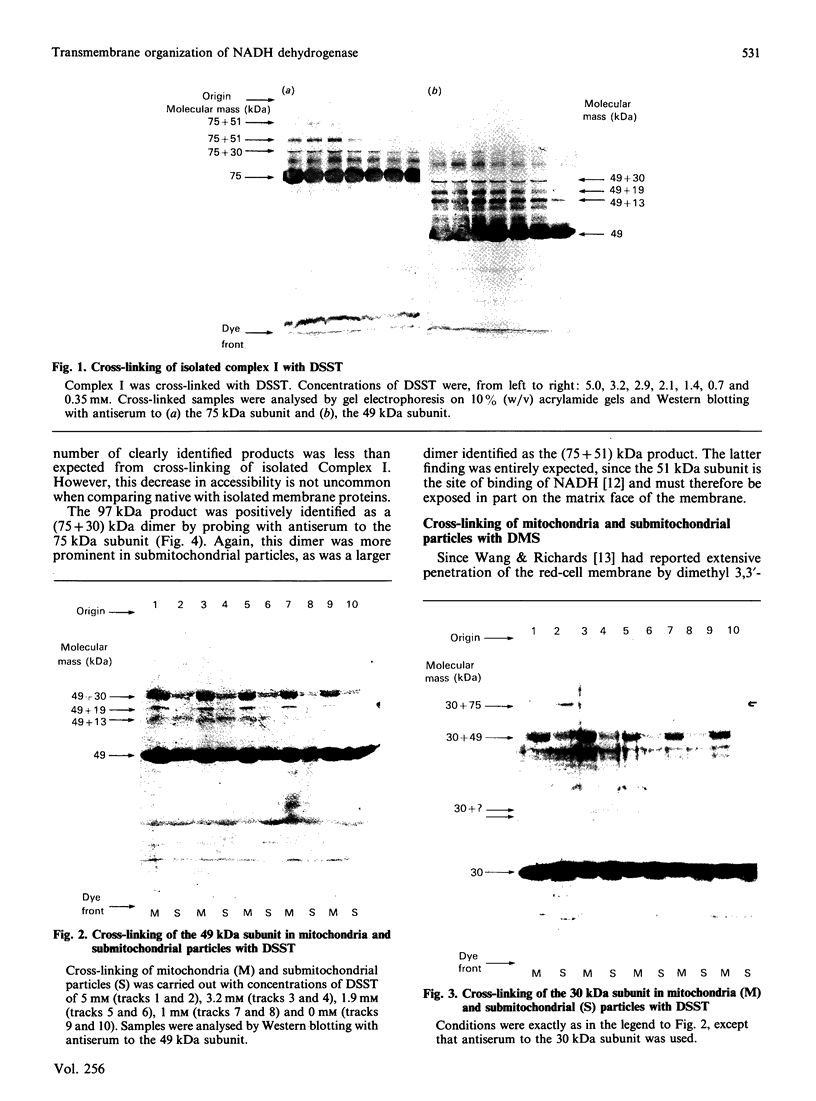
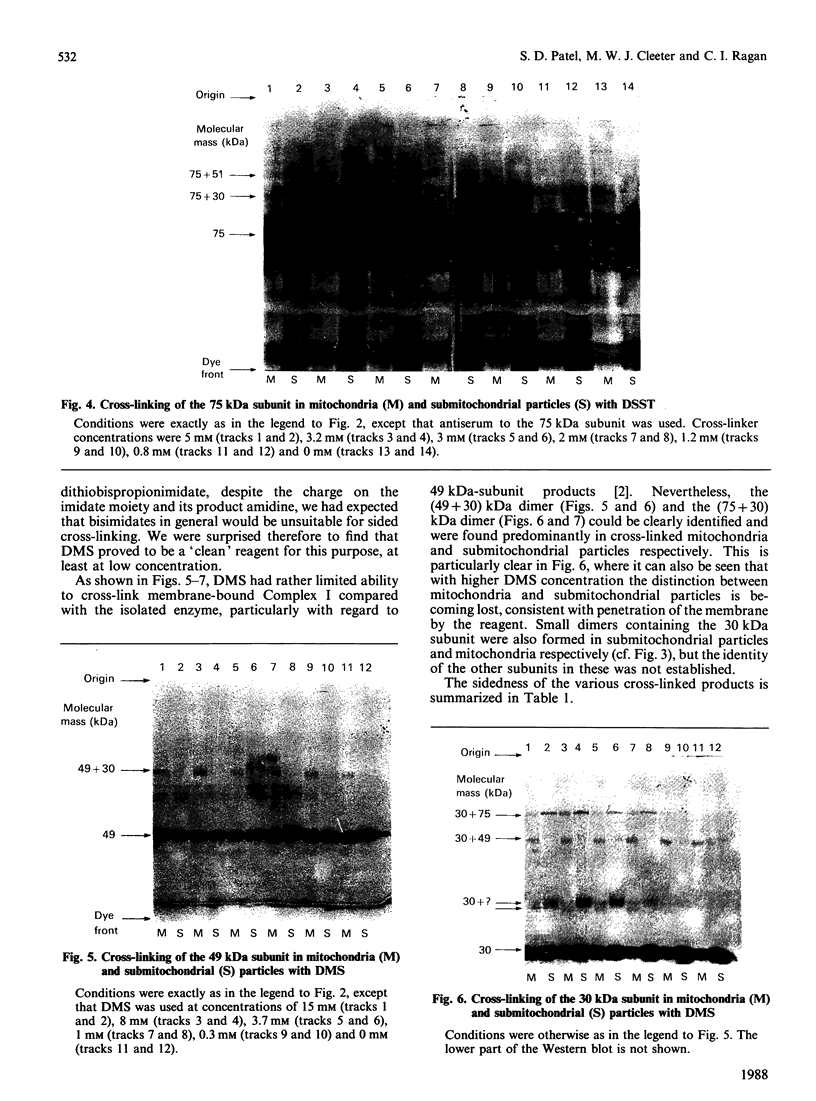
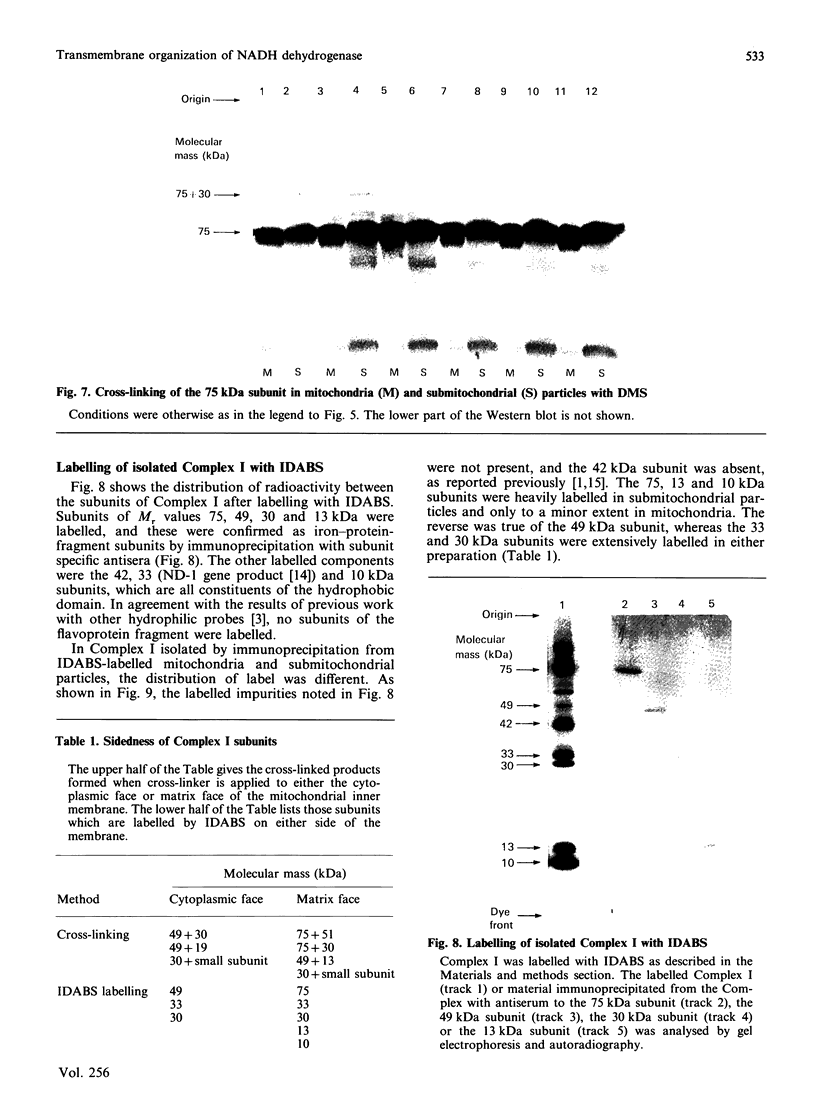
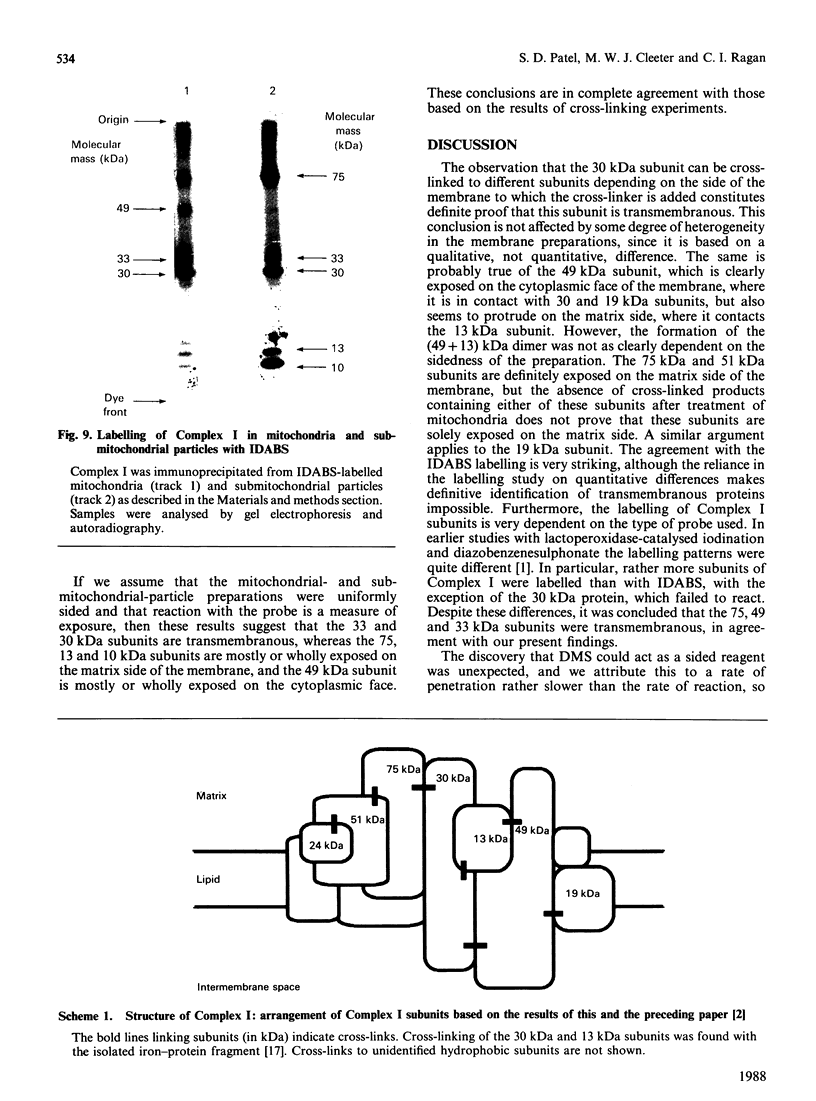
The organization of bovine heart NADH dehydrogenase in the mitochondrial inner membrane was investigated by chemical cross-linking and radiolabelling with [125I]iododiazobenzenesulphonate (IDABS). Mitochondria or submitochondrial particles were cross-linked with disulphosuccinimidyl tartrate and dimethyl suberimidate, and dimeric products containing subunits of the NADH dehydrogenase were analysed by Western blotting with subunit-specific antisera. Cross-linking of mitochondria gave rise to (49 + 30) kDa and (49 + 19) kDa dimers and an additional dimer containing the 30 kDa subunit. Cross-linking of submitochondrial particles gave rise to (75 + 51) kDa, (75 + 30) kDa and (49 + 13) kDa dimers and a further dimer containing the 30 kDa subunit. We conclude that the 49 kDa and 30 kDa subunits are transmembranous, the 19 kDa subunit is exposed on the cytoplasmic face of the membrane, whereas the 75, 51 and 13 kDa subunits are exposed on the matrix face of the membrane. Reaction of the isolated enzyme with IDABS results in labelling of 75, 49, 42, 33, 30, 13 and 10 kDa subunits. From experiments in which mitochondria or submitochondrial particles were first labelled and NADH dehydrogenase then isolated by immunoprecipitation, it was found that labelling of the 49 kDa subunit occurs predominantly from the cytoplasmic side of the membrane. On the other hand, labelling of the 75, 13 and 10 kDa subunits occurs predominantly from the matrix side of the membrane, whereas the 30 and 33 kDa subunits are heavily labelled from either side. These findings are consistent with those obtained from cross-linking.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRANE F. L., GLENN J. L., GREEN D. E. Studies on the electron transfer system. IV. The electron transfer particle. Biochim Biophys Acta. 1956 Dec;22(3):475–487. doi: 10.1016/0006-3002(56)90058-0. [DOI] [PubMed] [Google Scholar]
- Chen S., Guillory R. J. Studies on the interaction of arylazido-beta-alanyl NAD+ with the mitochondrial NADH dehydrogenase. J Biol Chem. 1981 Aug 25;256(16):8318–8323. [PubMed] [Google Scholar]
- Cleeter M. W., Banister S. H., Ragan C. I. Chemical cross-linking of mitochondrial NADH dehydrogenase from bovine heart. Biochem J. 1985 Apr 15;227(2):467–474. doi: 10.1042/bj2270467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeter M. W., Ragan C. I. The polypeptide composition of the mitochondrial NADH: ubiquinone reductase complex from several mammalian species. Biochem J. 1985 Sep 15;230(3):739–746. doi: 10.1042/bj2300739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley F. G., Patel S. D., Ragan I., Attardi G. Photolabelling of a mitochondrially encoded subunit of NADH dehydrogenase with [3H]dihydrorotenone. FEBS Lett. 1987 Jul 13;219(1):108–112. doi: 10.1016/0014-5793(87)81200-0. [DOI] [PubMed] [Google Scholar]
- Earley F. G., Ragan C. I. Identification of the subunits of bovine heart mitochondrial NADH dehydrogenase that are exposed to the phospholipid bilayer by photo-labelling with 5-iodonaphth-1-yl azide. Biochem J. 1980 Nov 1;191(2):429–436. doi: 10.1042/bj1910429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y., Stempel K. E. Isolation and enzymatic properties of the mitochondrial reduced diphosphopyridine nucleotide dehydrogenase. J Biol Chem. 1969 May 10;244(9):2350–2357. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Patel S. D., Ragan C. I. Structural studies on mitochondrial NADH dehydrogenase using chemical cross-linking. Biochem J. 1988 Dec 1;256(2):521–528. doi: 10.1042/bj2560521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Studies of factors involved in oxidative phosphorylation. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1659–1663. doi: 10.1073/pnas.48.9.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Ragan C. I. The organization of NADH dehydrogenase polypeptides in the inner mitochondrial membrane. Biochem J. 1980 Feb 1;185(2):315–326. doi: 10.1042/bj1850315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staros J. V. N-hydroxysulfosuccinimide active esters: bis(N-hydroxysulfosuccinimide) esters of two dicarboxylic acids are hydrophilic, membrane-impermeant, protein cross-linkers. Biochemistry. 1982 Aug 17;21(17):3950–3955. doi: 10.1021/bi00260a008. [DOI] [PubMed] [Google Scholar]
- Wang K., Richards F. M. An approach to nearest neighbor analysis of membrane proteins. Application to the human erythrocyte membrane of a method employing cleavable cross-linkages. J Biol Chem. 1974 Dec 25;249(24):8005–8018. [PubMed] [Google Scholar]