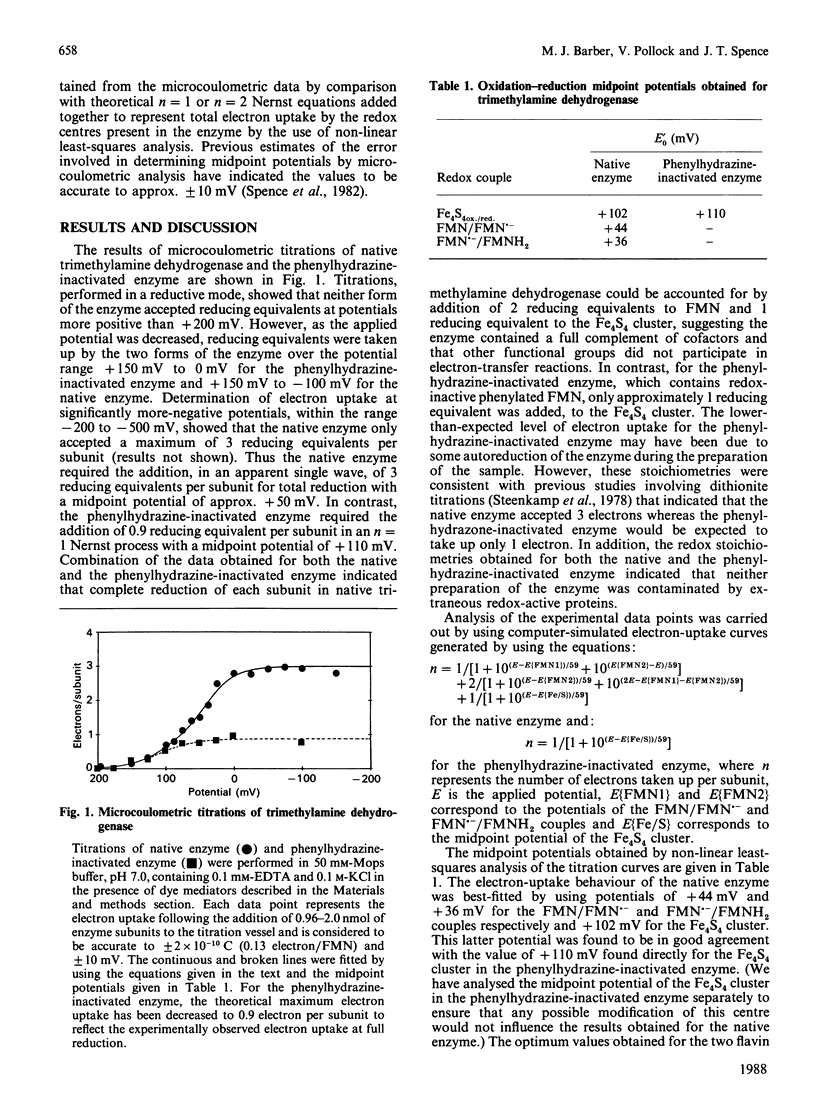
Abstract
Trimethylamine dehydrogenase, which contains one covalently bound 6-S-cysteinyl-FMN and one Fe4S4 cluster per subunit of molecular mass 83,000 Da, was purified to homogeneity from the methylotrophic bacterium W3A1. Microcoulometry at pH 7 in 50 mM-Mops buffer containing 0.1 mM-EDTA and 0.1 M-KCl revealed that the native enzyme required the addition of 3 reducing equivalents per subunit for complete reduction. In contrast, under identical conditions the phenylhydrazine-inhibited enzyme required the addition of 0.9 reducing equivalent per subunit with a midpoint potential of +110 mV. Least-squares analysis of the microcoulometric data obtained for the native enzyme, assuming uptake of 1 electron by Fe4S4 and 2 electrons by FMN, indicated midpoint potentials of +44 mV and +36 mV for the FMN/FMN.- and FMN.-/FMNH2 couples respectively and +102 mV for reduction of the Fe4S4 cluster.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colby J., Zatman L. J. Purification and properties of the trimethylamine dehydrogenase of bacterium 4B6. Biochem J. 1974 Dec;143(3):555–567. doi: 10.1042/bj1430555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. L., Steenkamp D. J., Holm R. H., Singer T. P. Identification of the iron-sulfur center in trimethylamine dehydrogenase. Proc Natl Acad Sci U S A. 1977 Feb;74(2):547–551. doi: 10.1073/pnas.74.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprzak A. A., Papas E. J., Steenkamp D. J. Identity of the subunits and the stoicheiometry of prosthetic groups in trimethylamine dehydrogenase and dimethylamine dehydrogenase. Biochem J. 1983 Jun 1;211(3):535–541. doi: 10.1042/bj2110535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L. W., Shamala N., Mathews F. S., Steenkamp D. J., Hamlin R., Xuong N. H. Three-dimensional structure of the iron-sulfur flavoprotein trimethylamine dehydrogenase at 2.4-A resolution. J Biol Chem. 1986 Nov 15;261(32):15140–15146. [PubMed] [Google Scholar]
- Müller F. The flavin redox-system and its biological function. Top Curr Chem. 1983;108:71–107. doi: 10.1007/3-540-11846-2_3. [DOI] [PubMed] [Google Scholar]
- Spence J. T., Barber M. J., Siegel L. M. Determination of the stoichiometry of electron uptake and the midpoint reduction potentials of milk xanthine oxidase at 25 degrees C by microcoulometry. Biochemistry. 1982 Mar 30;21(7):1656–1661. doi: 10.1021/bi00536a028. [DOI] [PubMed] [Google Scholar]
- Steenkamp D. J., Beinert H. Mechanistic studies on the dehydrogenases of methylotrophic bacteria. 1. The influence of substrate binding to reduced trimethylamine dehydrogenase on the intramolecular electron transfer between its prosthetic groups. Biochem J. 1982 Nov 1;207(2):233–239. doi: 10.1042/bj2070233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenkamp D. J., Beinert H. Mechanistic studies on the dehydrogenases of methylotrophic bacteria. 2. Kinetic studies on the intramolecular electron transfer in trimethylamine and dimethylamine dehydrogenase. Biochem J. 1982 Nov 1;207(2):241–252. doi: 10.1042/bj2070241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenkamp D. J., Mallinson J. Trimethylamine dehydrogenase from a methylotrophic bacterium. I. Isolation and steady-state kinetics. Biochim Biophys Acta. 1976 May 13;429(3):705–719. doi: 10.1016/0005-2744(76)90319-3. [DOI] [PubMed] [Google Scholar]
- Steenkamp D. J., Singer T. P. Participation of the iron-sulphur cluster and of the covalently bound coenzyme of trimethylamine dehydrogenase in catalysis. Biochem J. 1978 Feb 1;169(2):361–369. doi: 10.1042/bj1690361. [DOI] [PMC free article] [PubMed] [Google Scholar]


