Abstract
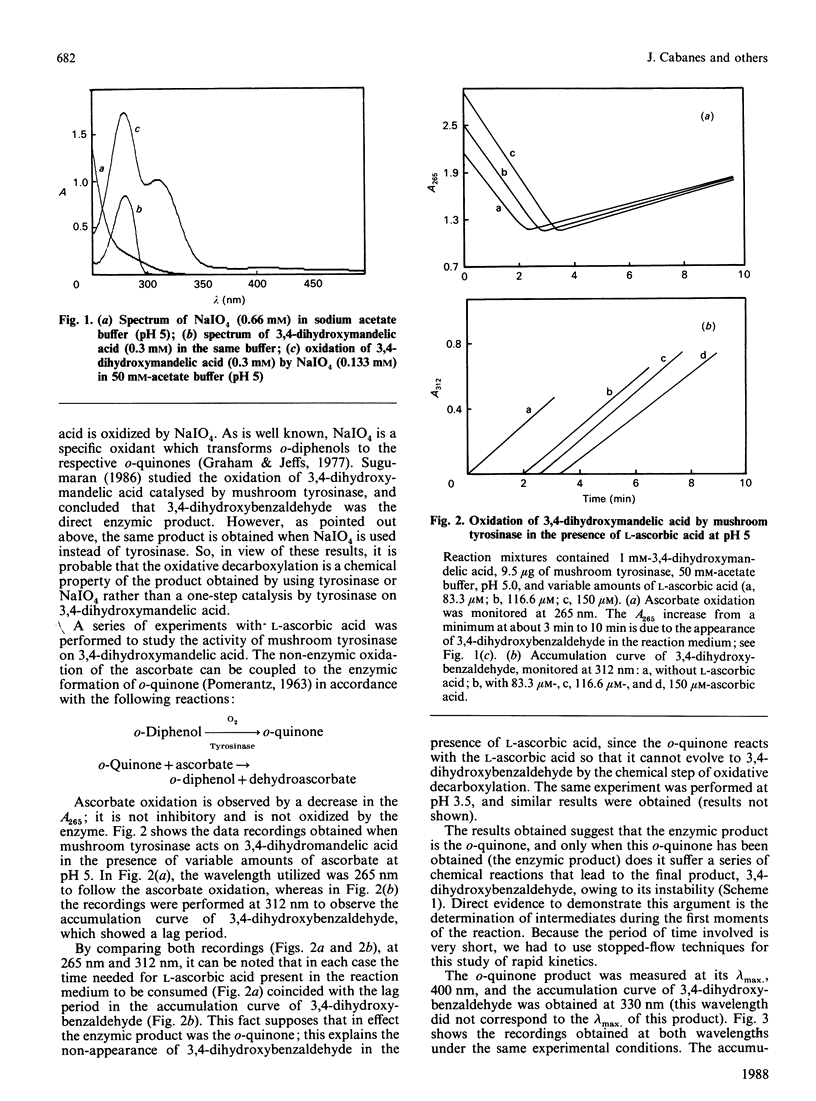
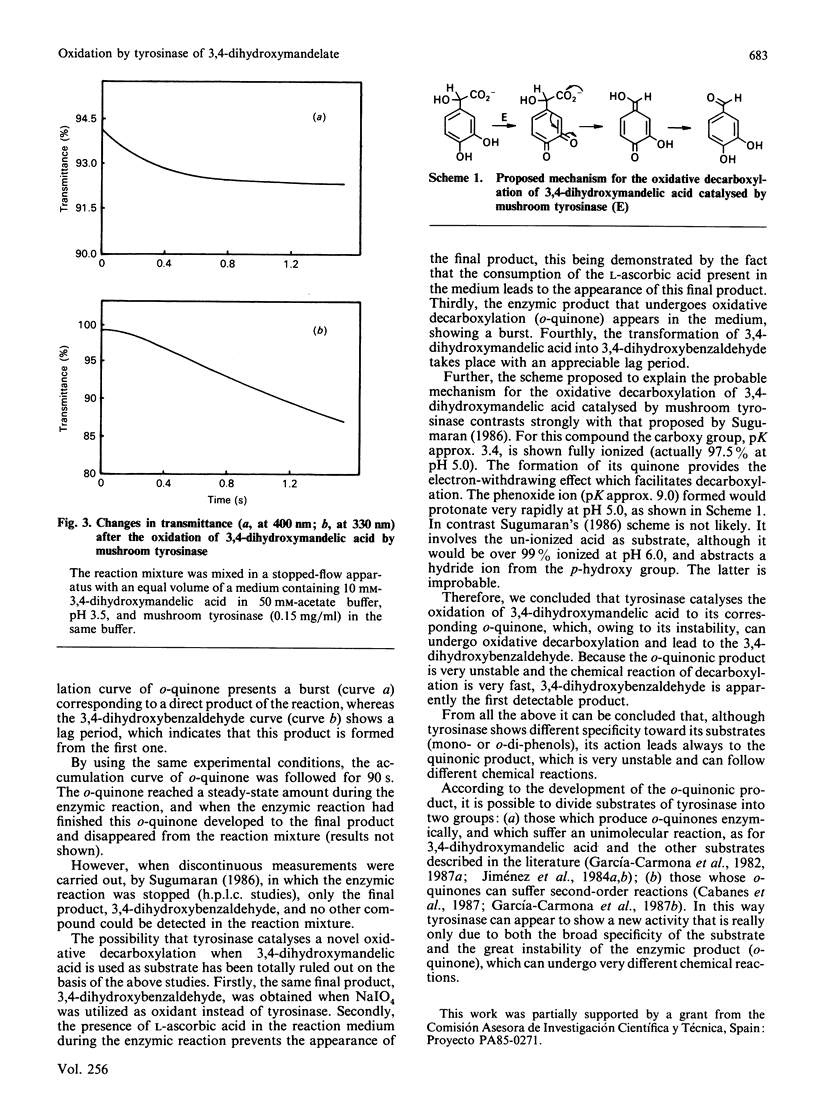
Tyrosinase usually catalyses the conversion of monophenols into o-diphenols and the oxidation of diphenols to the corresponding o-quinones. Sugumaran [(1986) Biochemistry 25, 4489-4492] has previously proposed an unusual oxidative decarboxylation of 3,4-dihydroxymandelate catalysed by tyrosinase. Our determination of the intermediates involved in the reaction demonstrated that 3,4-dihydroxybenzaldehyde is not the first intermediate appearing in the medium during the enzymic reaction. Re-examination of this new activity of tyrosinase has demonstrated that the product of the enzyme action is the o-quinone, which, owing to its instability, evolves to the final product, 3,4-dihydroxybenzaldehyde, by a chemical reaction of oxidative decarboxylation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cabanes J., García-Cánovas F., García-Carmona F. Chemical and enzymatic oxidation of 4-methylcatechol in the presence and absence of L-serine. Spectrophotometric determination of intermediates. Biochim Biophys Acta. 1987 Aug 5;914(2):190–197. doi: 10.1016/0167-4838(87)90063-x. [DOI] [PubMed] [Google Scholar]
- Cánovas F. G., García-Carmona F., Sánchez J. V., Pastor J. L., Teruel J. A. The role of pH in the melanin biosynthesis pathway. J Biol Chem. 1982 Aug 10;257(15):8738–8744. [PubMed] [Google Scholar]
- Garcia-Carmona F., Cabanes J., Garcia-Canovas F. Kinetic study of sinephrine oxidation by mushroom tyrosinase. Biochem Int. 1987 Jun;14(6):1003–1013. [PubMed] [Google Scholar]
- García-Carmona F., Cabanes J., García-Cánovas F. Enzymatic oxidation by frog epidermis tyrosinase of 4-methylcatechol and p-cresol. Influence of L-serine. Biochim Biophys Acta. 1987 Aug 5;914(2):198–204. doi: 10.1016/0167-4838(87)90064-1. [DOI] [PubMed] [Google Scholar]
- Graham D. G., Jeffs P. W. The role of 2,4,5-trihydroxyphenylalanine in melanin biosynthesis. J Biol Chem. 1977 Aug 25;252(16):5729–5734. [PubMed] [Google Scholar]
- Hansson C., Rorsman H., Rosengren E. 5-hydroxydopa, a new compound in the Raper-Mason scheme of melanogenesis. Acta Derm Venereol. 1980;60(4):281–286. [PubMed] [Google Scholar]
- Jimenez M., Garcia-Canovas F., Garcia-Carmona F., Lozano J. A., Iborra J. L. Kinetic study and intermediates identification of noradrenaline oxidation by tyrosinase. Biochem Pharmacol. 1984 Nov 15;33(22):3689–3697. doi: 10.1016/0006-2952(84)90158-8. [DOI] [PubMed] [Google Scholar]
- Jimenez M., Garcia-Carmona F., Garcia-Canovas F., Iborra J. L., Lozano J. A., Martinez F. Chemical intermediates in dopamine oxidation by tyrosinase, and kinetic studies of the process. Arch Biochem Biophys. 1984 Dec;235(2):438–448. doi: 10.1016/0003-9861(84)90217-0. [DOI] [PubMed] [Google Scholar]
- POMERANTZ S. H. Separation, purification, and properties of two tyrosinases from hamster melanoma. J Biol Chem. 1963 Jul;238:2351–2357. [PubMed] [Google Scholar]
- Sugumaran M. Tyrosinase catalyzes an unusual oxidative decarboxylation of 3,4-dihydroxymandelate. Biochemistry. 1986 Aug 12;25(16):4489–4492. doi: 10.1021/bi00364a005. [DOI] [PubMed] [Google Scholar]


