Abstract
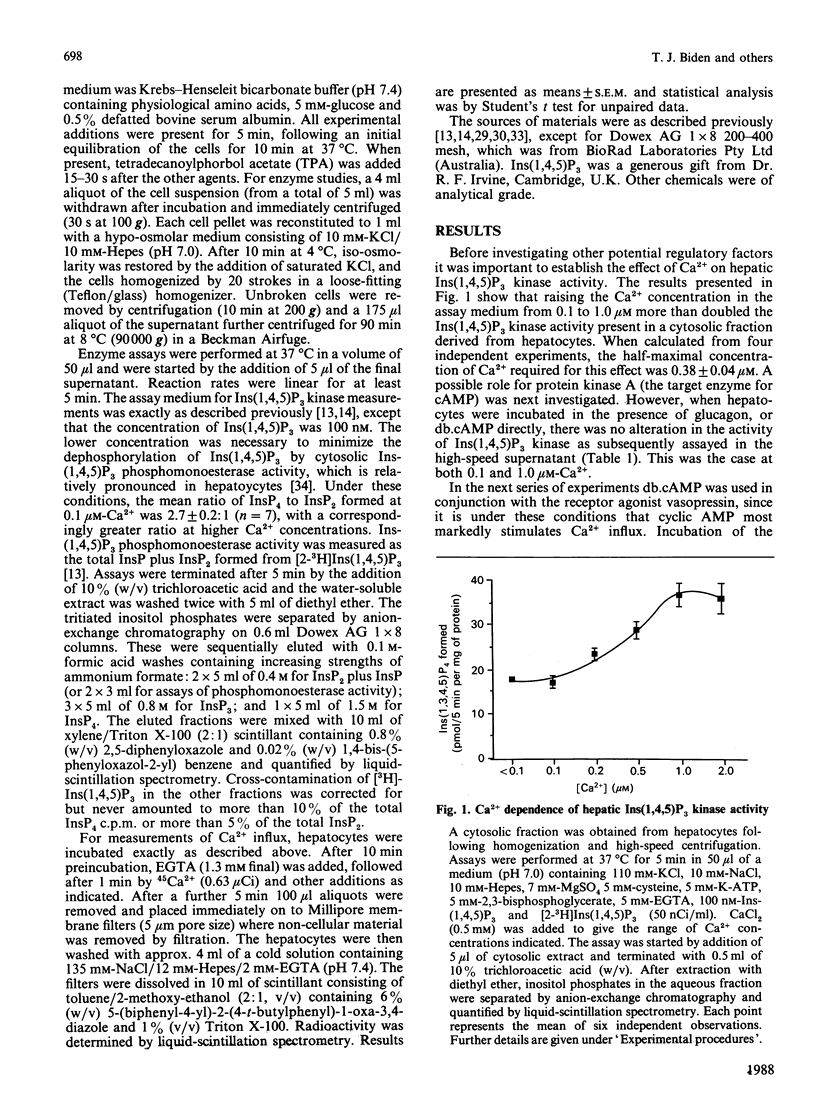
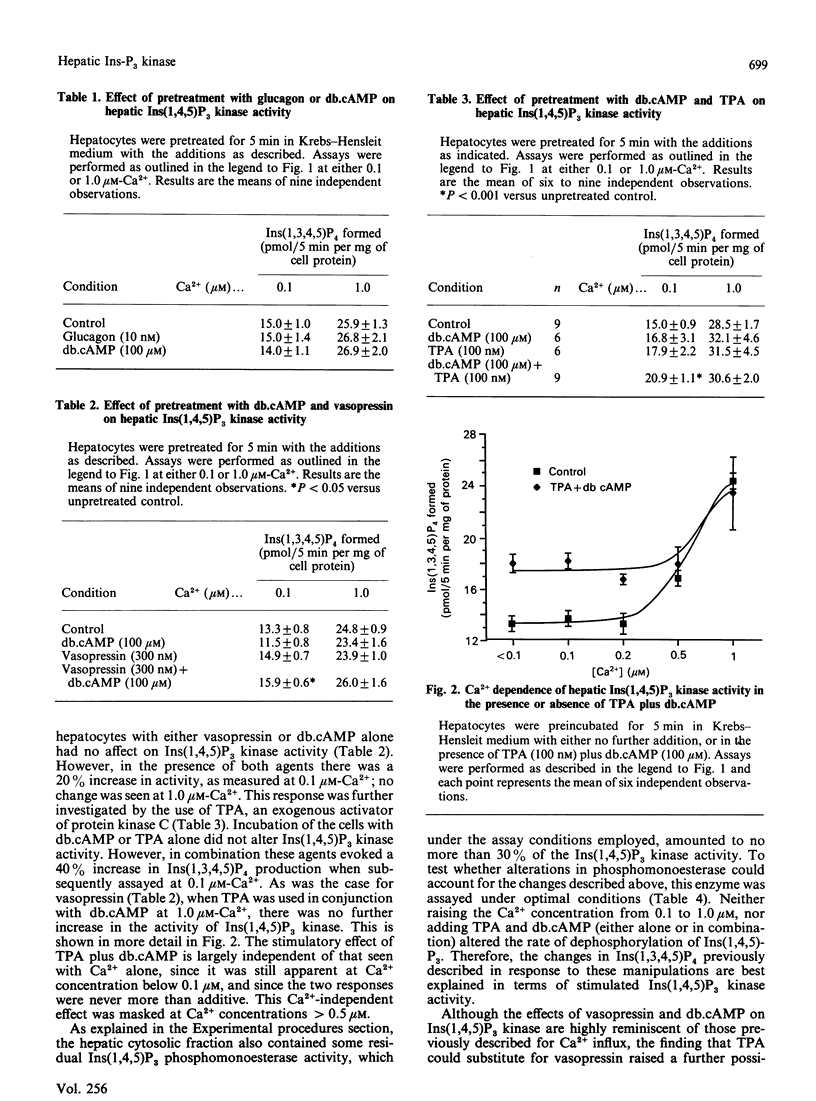
A cytosolic fraction derived from rat hepatocytes was used to investigate the regulation of inositol 1,4,5-trisphosphate [Ins(1,4,5)P3] kinase, the enzyme which converts Ins(1,4,5)P3 to inositol 1,3,4,5-tetrakisphosphate [Ins(1,3,4,5)P4]. The activity was doubled by raising the free Ca2+ concentration of the assay medium from 0.1 microM to 1.0 microM. A 5 min preincubation of the hepatocytes with 100 microM-dibutyryl cyclic AMP (db.cAMP) plus 100 nM-tetradecanoylphorbol acetate (TPA) resulted in a 40% increase in Ins(1,4,5)P3 kinase activity when subsequently assayed at 0.1 microM-Ca2+. This effect was smaller at [Ca2+] greater than 0.5 microM, and absent at 1.0 microM-Ca2+. Similar results were obtained after preincubation with 100 microM-db.cAMP plus 300 nM-vasopressin (20% increase at 0.1 microM-Ca2+; no effect at 1.0 microM-Ca2+). Preincubation with vasopressin, db.cAMP or TPA alone did not alter Ins(1,4,5)P3 kinase activity. It is proposed that these results, together with recent evidence implicating Ins(1,3,4,5)P4 in the control of Ca2+ influx, could be relevant to earlier findings that hepatic Ca2+ uptake is synergistically stimulated by cyclic AMP analogues and vasopressin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altin J. G., Bygrave F. L. Prostaglandin F2 alpha and the thromboxane A2 analogue ONO-11113 stimulate Ca2+ fluxes and other physiological responses in rat liver. Further evidence that prostanoids may be involved in the action of arachidonic acid and platelet-activating factor. Biochem J. 1988 Feb 1;249(3):677–685. doi: 10.1042/bj2490677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altin J. G., Bygrave F. L. Synergistic stimulation of Ca2+ uptake by glucagon and Ca2+-mobilizing hormones in the perfused rat liver. A role for mitochondria in long-term Ca2+ homoeostasis. Biochem J. 1986 Sep 15;238(3):653–661. doi: 10.1042/bj2380653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altin J. G., Bygrave F. L. The influx of Ca2+ induced by the administration of glucagon and Ca2+-mobilizing agents to the perfused rat liver could involve at least two separate pathways. Biochem J. 1987 Feb 15;242(1):43–50. doi: 10.1042/bj2420043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Comte M., Cox J. A., Wollheim C. B. Calcium-calmodulin stimulates inositol 1,4,5-trisphosphate kinase activity from insulin-secreting RINm5F cells. J Biol Chem. 1987 Jul 15;262(20):9437–9440. [PubMed] [Google Scholar]
- Biden T. J., Vallar L., Wollheim C. B. Regulation of inositol 1,4,5-trisphosphate metabolism in insulin-secreting RINm5F cells. Biochem J. 1988 Apr 15;251(2):435–440. doi: 10.1042/bj2510435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B. Ca2+ regulates the inositol tris/tetrakisphosphate pathway in intact and broken preparations of insulin-secreting RINm5F cells. J Biol Chem. 1986 Sep 15;261(26):11931–11934. [PubMed] [Google Scholar]
- Blackmore P. F., Exton J. H. Studies on the hepatic calcium-mobilizing activity of aluminum fluoride and glucagon. Modulation by cAMP and phorbol myristate acetate. J Biol Chem. 1986 Aug 25;261(24):11056–11063. [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Berthon B., Exton J. H. Changes in free cytosolic Ca2+ in hepatocytes following alpha 1-adrenergic stimulation. Studies on Quin-2-loaded hepatocytes. J Biol Chem. 1983 Jul 25;258(14):8769–8773. [PubMed] [Google Scholar]
- Cocks T. M., Jenkinson D. H., Koller K. Interactions between receptors that increase cytosolic calcium and cyclic AMP in guinea-pig liver cells. Br J Pharmacol. 1984 Sep;83(1):281–291. doi: 10.1111/j.1476-5381.1984.tb10144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combettes L., Berthon B., Binet A., Claret M. Glucagon and vasopressin interactions on Ca2+ movements in isolated hepatocytes. Biochem J. 1986 Aug 1;237(3):675–683. doi: 10.1042/bj2370675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T. M., Bansal V. S., Bross T. E., Irvine R. F., Majerus P. W. The metabolism of tris- and tetraphosphates of inositol by 5-phosphomonoesterase and 3-kinase enzymes. J Biol Chem. 1987 Feb 15;262(5):2146–2149. [PubMed] [Google Scholar]
- Connolly T. M., Lawing W. J., Jr, Majerus P. W. Protein kinase C phosphorylates human platelet inositol trisphosphate 5'-phosphomonoesterase, increasing the phosphatase activity. Cell. 1986 Sep 12;46(6):951–958. doi: 10.1016/0092-8674(86)90077-2. [DOI] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms involved in alpha-adrenergic phenomena. Am J Physiol. 1985 Jun;248(6 Pt 1):E633–E647. doi: 10.1152/ajpendo.1985.248.6.E633. [DOI] [PubMed] [Google Scholar]
- Hansen C. A., Mah S., Williamson J. R. Formation and metabolism of inositol 1,3,4,5-tetrakisphosphate in liver. J Biol Chem. 1986 Jun 25;261(18):8100–8103. [PubMed] [Google Scholar]
- Hepler J. R., Earp H. S., Harden T. K. Long-term phorbol ester treatment down-regulates protein kinase C and sensitizes the phosphoinositide signaling pathway to hormone and growth factor stimulation. Evidence for a role of protein kinase C in agonist-induced desensitization. J Biol Chem. 1988 Jun 5;263(16):7610–7619. [PubMed] [Google Scholar]
- Imboden J. B., Pattison G. Regulation of inositol 1,4,5-trisphosphate kinase activity after stimulation of human T cell antigen receptor. J Clin Invest. 1987 May;79(5):1538–1541. doi: 10.1172/JCI112986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden J. B., Pattison G. Regulation of inositol 1,4,5-trisphosphate kinase activity after stimulation of human T cell antigen receptor. J Clin Invest. 1987 May;79(5):1538–1541. doi: 10.1172/JCI112986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Inositol(1,3,4,5)tetrakisphosphate-induced activation of sea urchin eggs requires the presence of inositol trisphosphate. Biochem Biophys Res Commun. 1987 Jul 15;146(1):284–290. doi: 10.1016/0006-291x(87)90723-6. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Coll K. E., Thomas A. P., Rubin R., Williamson J. R. The role of extracellular Ca2+ in the response of the hepatocyte to Ca2+-dependent hormones. J Biol Chem. 1985 Oct 15;260(23):12508–12515. [PubMed] [Google Scholar]
- Joseph S. K., Hansen C. A., Williamson J. R. Inositol 1,3,4,5-tetrakisphosphate increases the duration of the inositol 1,4,5-trisphosphate-mediated Ca2+ transient. FEBS Lett. 1987 Jul 13;219(1):125–129. doi: 10.1016/0014-5793(87)81203-6. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Claret M. Synergistic stimulation of the Ca2+ influx in rat hepatocytes by glucagon and the Ca2+-linked hormones vasopressin and angiotensin II. J Biol Chem. 1985 Sep 25;260(21):11635–11642. [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Guesdon F., Claret M. Noradrenaline, vasopressin and angiotensin increase Ca2+ influx by opening a common pool of Ca2+ channels in isolated rat liver cells. Biochem J. 1984 Jul 1;221(1):121–127. doi: 10.1042/bj2210121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N. G., Blackmore P. F., Exton J. H. Modulation of the alpha 1-adrenergic control of hepatocyte calcium redistribution by increases in cyclic AMP. J Biol Chem. 1983 Apr 25;258(8):5110–5116. [PubMed] [Google Scholar]
- Morris A. J., Downes C. P., Harden T. K., Michell R. H. Turkey erythrocytes possess a membrane-associated inositol 1,4,5-trisphosphate 3-kinase that is activated by Ca2+ in the presence of calmodulin. Biochem J. 1987 Dec 1;248(2):489–493. doi: 10.1042/bj2480489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Orellana S., Solski P. A., Brown J. H. Guanosine 5'-O-(thiotriphosphate)-dependent inositol trisphosphate formation in membranes is inhibited by phorbol ester and protein kinase C. J Biol Chem. 1987 Feb 5;262(4):1638–1643. [PubMed] [Google Scholar]
- Poggioli J., Mauger J. P., Claret M. Effect of cyclic AMP-dependent hormones and Ca2+-mobilizing hormones on the Ca2+ influx and polyphosphoinositide metabolism in isolated rat hepatocytes. Biochem J. 1986 May 1;235(3):663–669. doi: 10.1042/bj2350663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H. The calcium messenger system (1). N Engl J Med. 1986 Apr 24;314(17):1094–1101. doi: 10.1056/NEJM198604243141707. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. The role of calcium ions in the mechanism of action of alpha-adrenergic agonists in rat liver. Biochem J. 1984 Oct 1;223(1):1–13. doi: 10.1042/bj2230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard D., Poggioli J. Does the inositol tris/tetrakisphosphate pathway exist in rat heart? FEBS Lett. 1987 Jun 8;217(1):117–123. doi: 10.1016/0014-5793(87)81254-1. [DOI] [PubMed] [Google Scholar]
- Ryu S. H., Lee S. Y., Lee K. Y., Rhee S. G. Catalytic properties of inositol trisphosphate kinase: activation by Ca2+ and calmodulin. FASEB J. 1987 Nov;1(5):388–393. doi: 10.1096/fasebj.1.5.2824270. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Uhing R. J., Snyderman R. Nucleotide regulatory protein-mediated activation of phospholipase C in human polymorphonuclear leukocytes is disrupted by phorbol esters. J Biol Chem. 1987 May 5;262(13):6121–6127. [PubMed] [Google Scholar]
- Thomas A. P., Alexander J., Williamson J. R. Relationship between inositol polyphosphate production and the increase of cytosolic free Ca2+ induced by vasopressin in isolated hepatocytes. J Biol Chem. 1984 May 10;259(9):5574–5584. [PubMed] [Google Scholar]
- Wakelam M. J., Murphy G. J., Hruby V. J., Houslay M. D. Activation of two signal-transduction systems in hepatocytes by glucagon. Nature. 1986 Sep 4;323(6083):68–71. doi: 10.1038/323068a0. [DOI] [PubMed] [Google Scholar]
- Whipps D. E., Armston A. E., Pryor H. J., Halestrap A. P. Effects of glucagon and Ca2+ on the metabolism of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in isolated rat hepatocytes and plasma membranes. Biochem J. 1987 Feb 1;241(3):835–845. doi: 10.1042/bj2410835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Joseph S. K., Thomas A. P. Inositol trisphosphate and diacylglycerol as intracellular second messengers in liver. Am J Physiol. 1985 Mar;248(3 Pt 1):C203–C216. doi: 10.1152/ajpcell.1985.248.3.C203. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Hansen C. A., Verhoeven A., Coll K. E., Johanson R., Williamson M. T., Filburn C. Mechanisms involved in receptor-mediated changes of intracellular Ca2+ in liver. Soc Gen Physiol Ser. 1987;42:93–116. [PubMed] [Google Scholar]
- Yamaguchi K., Hirata M., Kuriyama H. Calmodulin activates inositol 1,4,5-trisphosphate 3-kinase activity in pig aortic smooth muscle. Biochem J. 1987 Jun 15;244(3):787–791. doi: 10.1042/bj2440787. [DOI] [PMC free article] [PubMed] [Google Scholar]


