Abstract
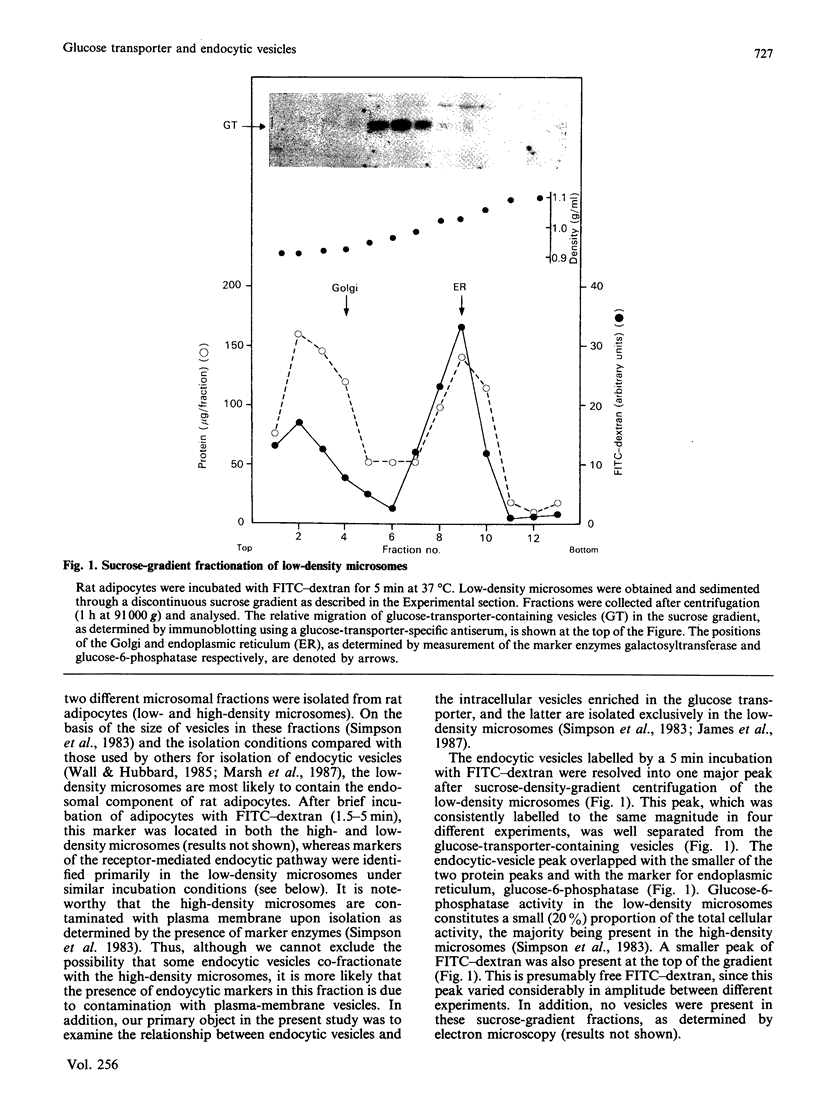
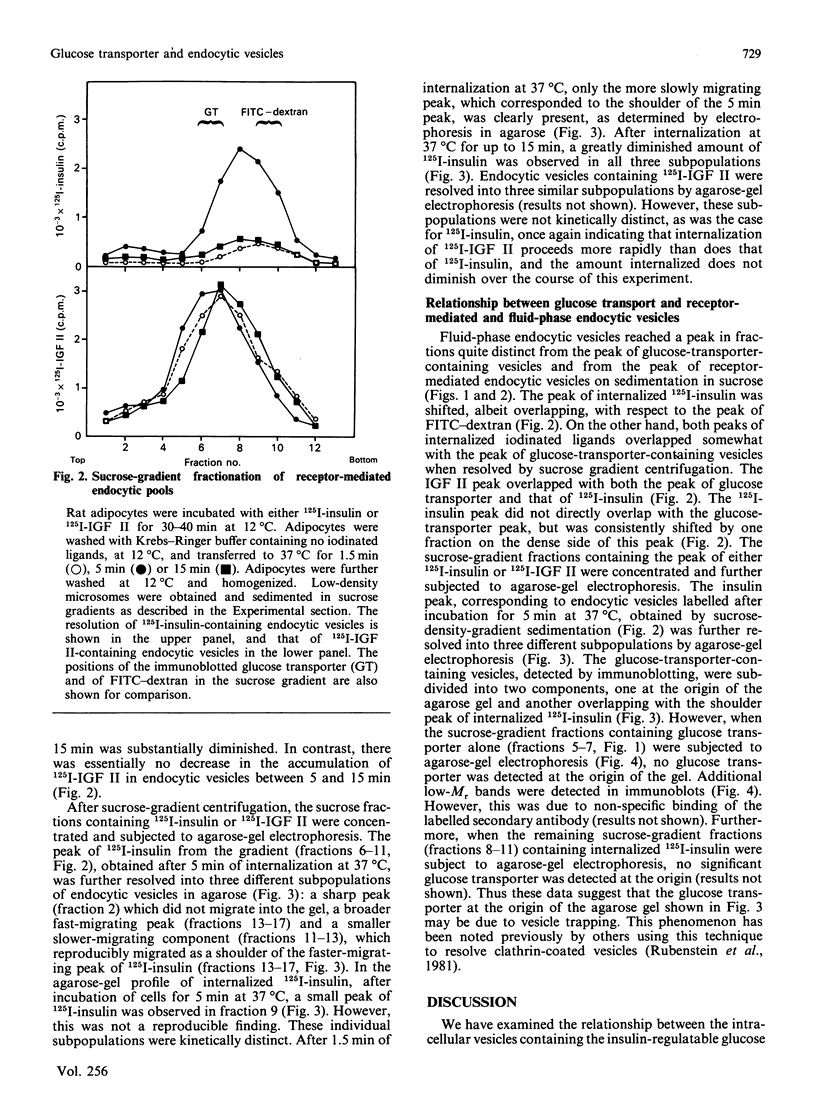
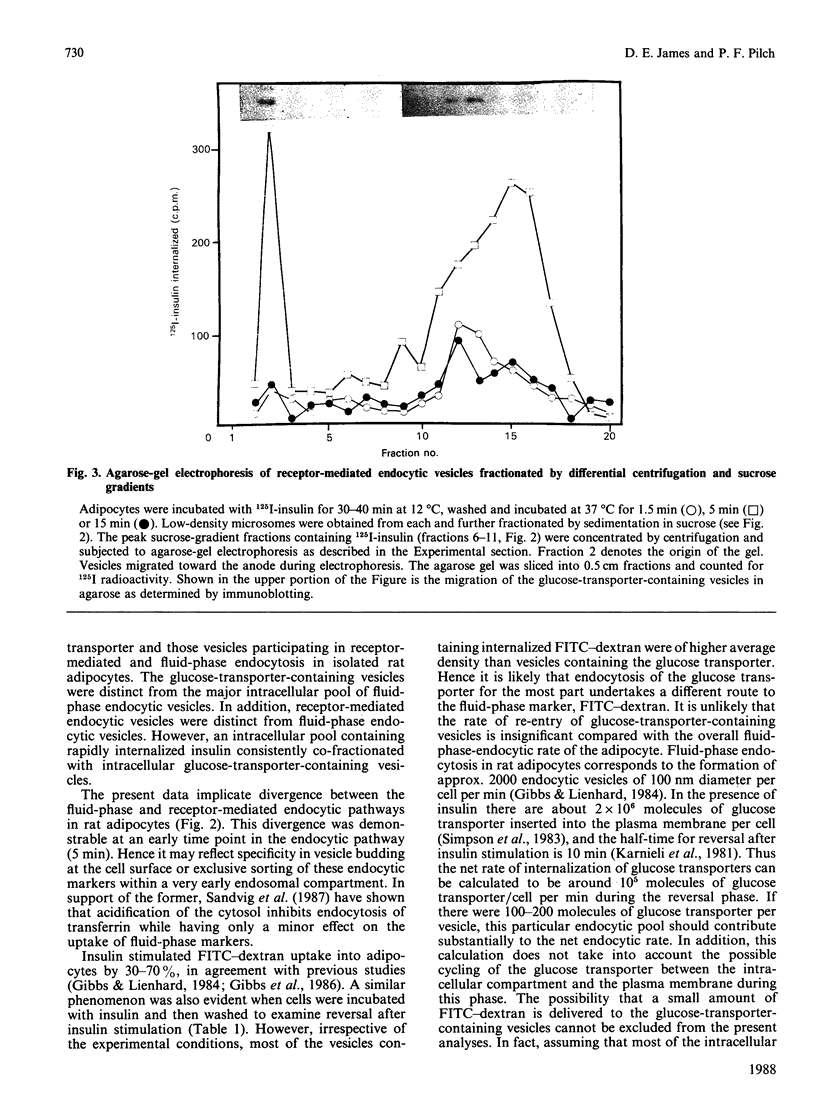
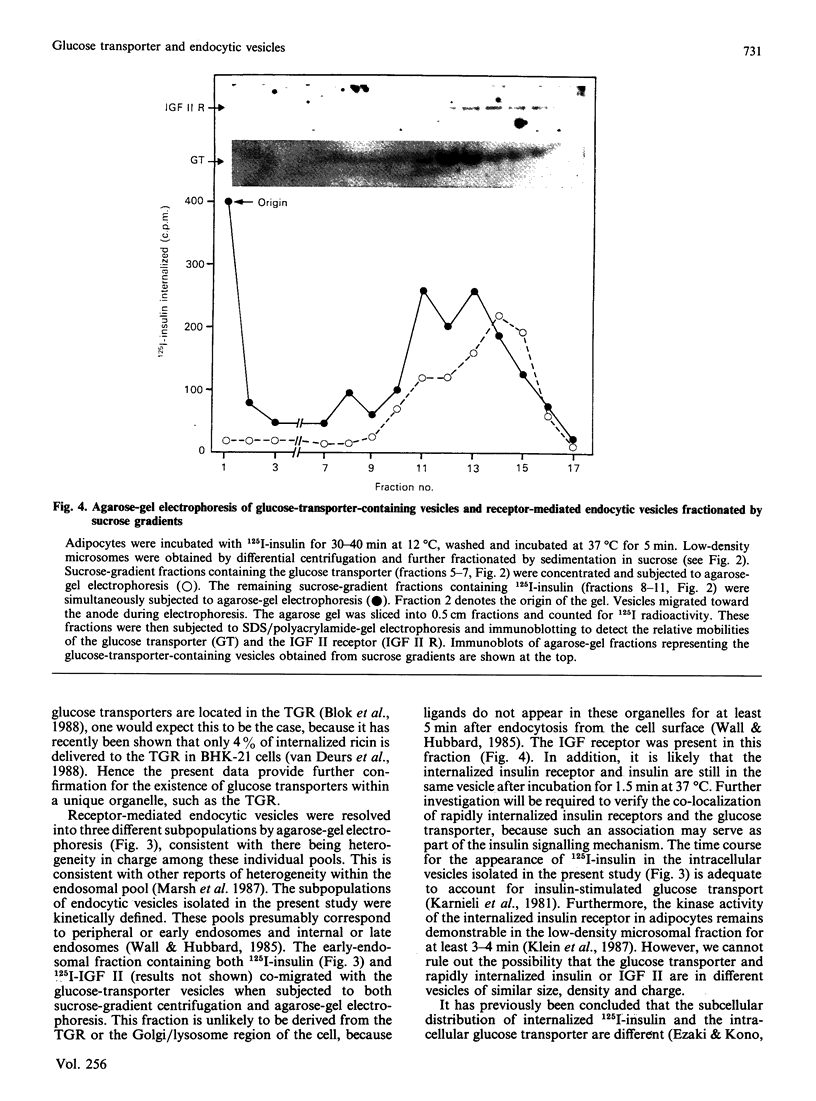
We subfractionated intracellular vesicles from rat adipocytes in order to examine the subcellular distribution of endocytic vesicles or endosomes with respect to insulin-regulatable glucose-transporter (GT)-containing vesicles [James, Lederman & Pilch (1987) J. Biol. Chem. 262, 11817-11824]. Vesicles mediating fluid-phase endocytosis sedimented as a single major peak of greater density than the single distinct peak of GT-containing vesicles. This difference was also apparent during cellular insulin exposure and after insulin removal. Endocytosis of insulin and IGF (insulin-like growth factor) II was also examined. In sucrose gradients, IGF II-containing vesicles were less dense than those containing internalized insulin. Receptor-mediated endocytic vesicles were distinct from fluid-phase endocytic vesicles, but overlapped with the GT-containing vesicles. Vesicles containing internalized ligand were further fractionated by agarose-gel electrophoresis after various times of internalization. At least three different vesicle subpopulations containing the iodinated ligands were resolved after 5 min of internalization. Endocytic vesicles containing rapidly internalized insulin (1.5 min at 37 degrees C) consistently co-migrated with GT-containing vesicles. These data indicate that fluid-phase and receptor-mediated endocytosis occur via different pathways in adipocytes. Furthermore, whereas the intracellular GT-containing vesicles are distinct from fluid-phase vesicles, a rapidly labelled pool of insulin-containing vesicles consistently co-fractionated with GT-containing vesicles when separation techniques based on size, density and charge were used. This suggests that the insulin receptor may directly interact with the intracellular GT-containing vesicles after insulin-induced endocytosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blok J., Gibbs E. M., Lienhard G. E., Slot J. W., Geuze H. J. Insulin-induced translocation of glucose transporters from post-Golgi compartments to the plasma membrane of 3T3-L1 adipocytes. J Cell Biol. 1988 Jan;106(1):69–76. doi: 10.1083/jcb.106.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman S. W. Structure-function relationships in the adipose cell. I. Ultrastructure of the isolated adipose cell. J Cell Biol. 1970 Aug;46(2):326–341. doi: 10.1083/jcb.46.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Ezaki O., Kono T. Sedimentation characteristics of subcellular vesicles associated with internalized insulin and those bound with intracellular glucose transport activity. Arch Biochem Biophys. 1984 Jun;231(2):280–286. doi: 10.1016/0003-9861(84)90389-8. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Lodish H. F., Schwartz A. L. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983 Jan;32(1):277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Gibbs E. M., Lienhard G. E., Appleman J. R., Lane M. D., Frost S. C. Insulin stimulates fluid-phase endocytosis and exocytosis in 3T3-L1 adipocytes. J Biol Chem. 1986 Mar 25;261(9):3944–3951. [PubMed] [Google Scholar]
- Gibbs E. M., Lienhard G. E. Fluid-phase endocytosis by isolated rat adipocytes. J Cell Physiol. 1984 Dec;121(3):569–575. doi: 10.1002/jcp.1041210316. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Osterlind K., Vinten J., Gammeltoft S. A procedure for measurement of distribution spaces in isolated fat cells. Biochim Biophys Acta. 1972 Nov 24;286(1):1–9. doi: 10.1016/0304-4165(72)90082-7. [DOI] [PubMed] [Google Scholar]
- James D. E., Lederman L., Pilch P. F. Purification of insulin-dependent exocytic vesicles containing the glucose transporter. J Biol Chem. 1987 Aug 25;262(24):11817–11824. [PubMed] [Google Scholar]
- Karnieli E., Zarnowski M. J., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981 May 25;256(10):4772–4777. [PubMed] [Google Scholar]
- Klein H. H., Freidenberg G. R., Matthaei S., Olefsky J. M. Insulin receptor kinase following internalization in isolated rat adipocytes. J Biol Chem. 1987 Aug 5;262(22):10557–10564. [PubMed] [Google Scholar]
- Koldovský O., Palmieri M. Cortisone-evoked decrease of acid -galactosidase, -glucuronidase, N-acetyl- -glucosaminidase and arylsulphatase in the ileum of suckling rats. Biochem J. 1971 Dec;125(3):697–701. doi: 10.1042/bj1250697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono T., Suzuki K., Dansey L. E., Robinson F. W., Blevins T. L. Energy-dependent and protein synthesis-independent recycling of the insulin-sensitive glucose transport mechanism in fat cells. J Biol Chem. 1981 Jun 25;256(12):6400–6407. [PubMed] [Google Scholar]
- Lienhard G. E., Kim H. H., Ransome K. J., Gorga J. C. Immunological identification of an insulin-responsive glucose transporter. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1150–1156. doi: 10.1016/0006-291x(82)91090-7. [DOI] [PubMed] [Google Scholar]
- Marsh M., Schmid S., Kern H., Harms E., Male P., Mellman I., Helenius A. Rapid analytical and preparative isolation of functional endosomes by free flow electrophoresis. J Cell Biol. 1987 Apr;104(4):875–886. doi: 10.1083/jcb.104.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S. Degradative processing of internalized insulin in isolated adipocytes. J Biol Chem. 1985 Nov 5;260(25):13517–13523. [PubMed] [Google Scholar]
- Marshall S. Kinetics of insulin receptor internalization and recycling in adipocytes. Shunting of receptors to a degradative pathway by inhibitors of recycling. J Biol Chem. 1985 Apr 10;260(7):4136–4144. [PubMed] [Google Scholar]
- McKeel D. W., Jarett L. Preparation and characterization of a plasma membrane fraction from isolated fat cells. J Cell Biol. 1970 Feb;44(2):417–432. doi: 10.1083/jcb.44.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Morgan D. O., Edman J. C., Standring D. N., Fried V. A., Smith M. C., Roth R. A., Rutter W. J. Insulin-like growth factor II receptor as a multifunctional binding protein. Nature. 1987 Sep 24;329(6137):301–307. doi: 10.1038/329301a0. [DOI] [PubMed] [Google Scholar]
- Oka Y., Mottola C., Oppenheimer C. L., Czech M. P. Insulin activates the appearance of insulin-like growth factor II receptors on the adipocyte cell surface. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4028–4032. doi: 10.1073/pnas.81.13.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R. The endosomal concentration of a mannose 6-phosphate receptor is unchanged in the absence of ligand synthesis. J Cell Biol. 1987 Jul;105(1):229–234. doi: 10.1083/jcb.105.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rubenstein J. L., Fine R. E., Luskey B. D., Rothman J. E. Purification of coated vesicles by agarose gel electrophoresis. J Cell Biol. 1981 May;89(2):357–361. doi: 10.1083/jcb.89.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahagian G. G. The mannose 6-phosphate receptor: function, biosynthesis and translocation. Biol Cell. 1984;51(2):207–214. doi: 10.1111/j.1768-322x.1984.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Petersen O. W., van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987 Aug;105(2):679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson I. A., Yver D. R., Hissin P. J., Wardzala L. J., Karnieli E., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transporters in the isolated rat adipose cells: characterization of subcellular fractions. Biochim Biophys Acta. 1983 Dec 19;763(4):393–407. doi: 10.1016/0167-4889(83)90101-5. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Internalization and degradation of fat cell-bound insulin. Separation and partial characterization of subcellular vesicles associated with iodoinsulin. J Biol Chem. 1979 Oct 10;254(19):9786–9794. [PubMed] [Google Scholar]
- Wall D. A., Hubbard A. L. Receptor-mediated endocytosis of asialoglycoproteins by rat liver hepatocytes: biochemical characterization of the endosomal compartments. J Cell Biol. 1985 Dec;101(6):2104–2112. doi: 10.1083/jcb.101.6.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardzala L. J., Simpson I. A., Rechler M. M., Cushman S. W. Potential mechanism of the stimulatory action of insulin on insulin-like growth factor II binding to the isolated rat adipose cell. Apparent redistribution of receptors cycling between a large intracellular pool and the plasma membrane. J Biol Chem. 1984 Jul 10;259(13):8378–8383. [PubMed] [Google Scholar]
- Wheeler T. J., Simpson I. A., Sogin D. C., Hinkle P. C., Cushman S. W. Detection of the rat adipose cell glucose transporter with antibody against the human red cell glucose transporter. Biochem Biophys Res Commun. 1982 Mar 15;105(1):89–95. doi: 10.1016/s0006-291x(82)80014-4. [DOI] [PubMed] [Google Scholar]
- van Deurs B., Sandvig K., Petersen O. W., Olsnes S., Simons K., Griffiths G. Estimation of the amount of internalized ricin that reaches the trans-Golgi network. J Cell Biol. 1988 Feb;106(2):253–267. doi: 10.1083/jcb.106.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K., Gieselmann V., Hasilik A. Antibody to mannose 6-phosphate specific receptor induces receptor deficiency in human fibroblasts. EMBO J. 1984 Jun;3(6):1281–1286. doi: 10.1002/j.1460-2075.1984.tb01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]