Abstract
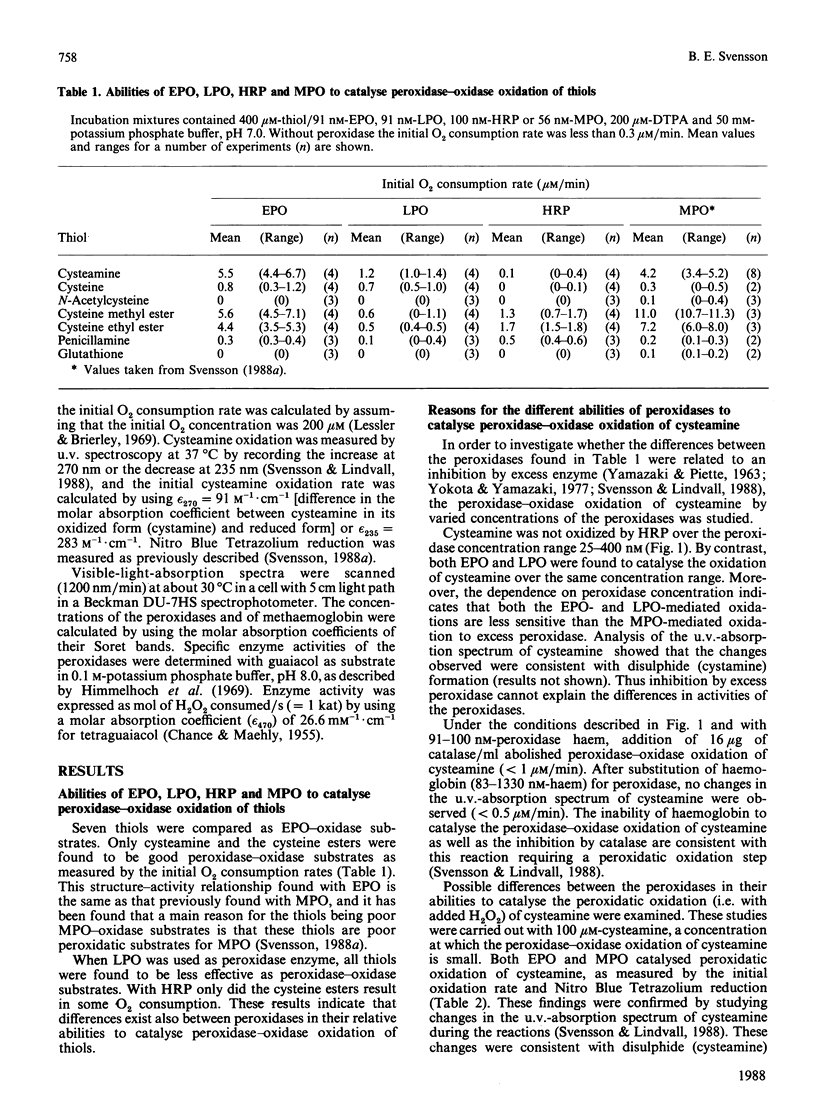
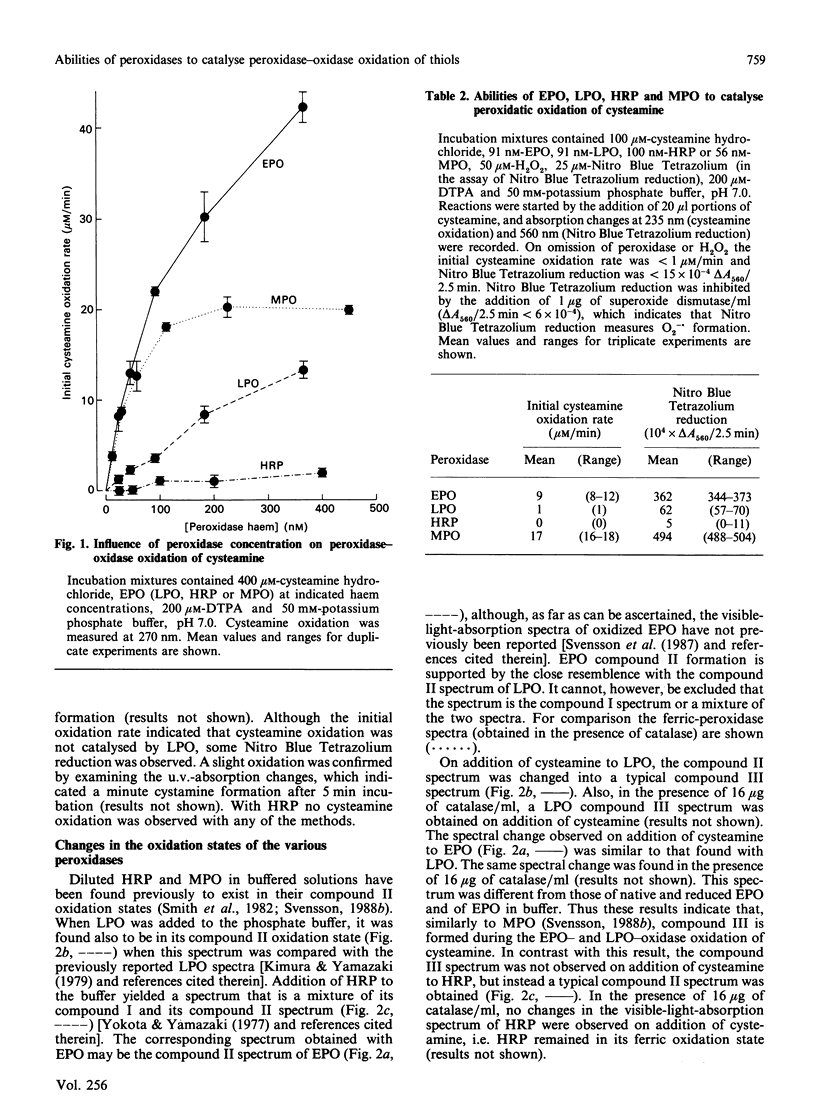
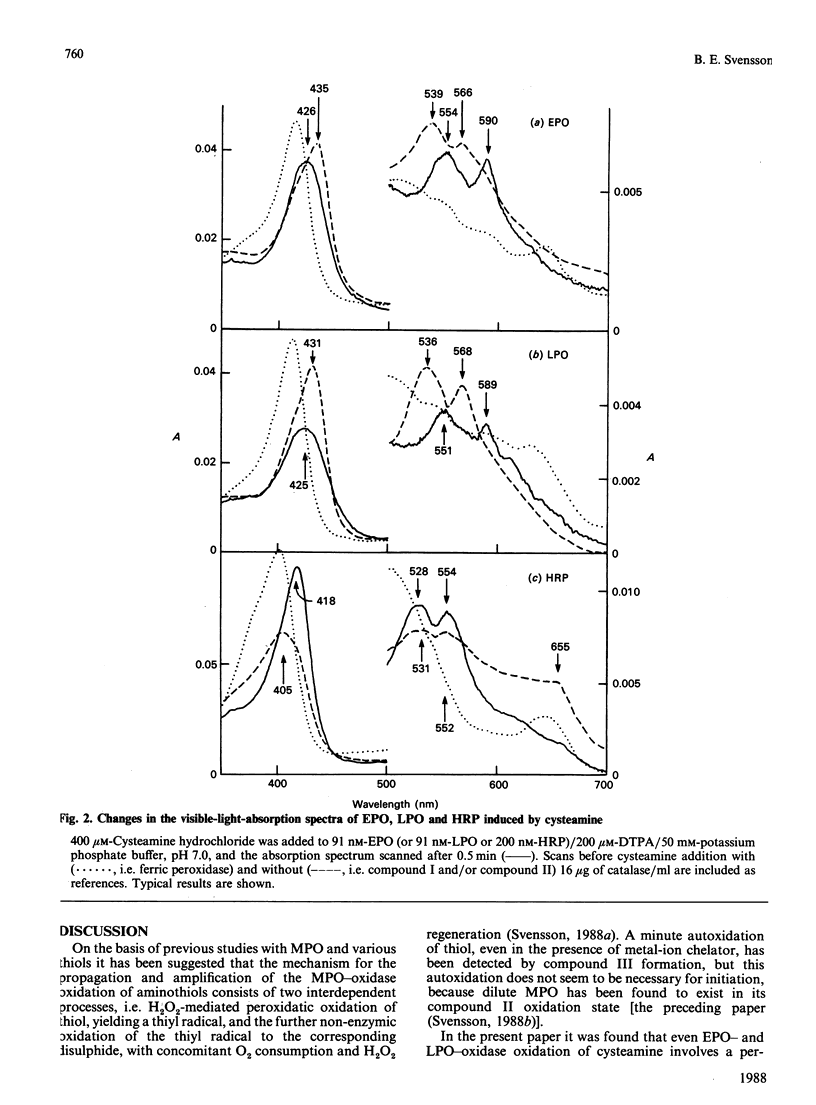
The abilities of various peroxidases to catalyse the peroxidase-oxidase oxidation of seven aminothiols were studied. Cysteamine and cysteine esters were found to be peroxidase-oxidase substrates for eosinophil peroxidase and myeloperoxidase, whereas other thiols tested were inactive or poorly active with these peroxidases. With lactoperoxidase and horseradish peroxidase, all the tested thiols were inactive or poorly active as peroxidase-oxidase substrates. These studies suggest that a main reason for thiols being poor peroxidase-oxidase substrates is because these thiols are poor peroxidatic substrates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agosti J. M., Altman L. C., Ayars G. H., Loegering D. A., Gleich G. J., Klebanoff S. J. The injurious effect of eosinophil peroxidase, hydrogen peroxide, and halides on pneumocytes in vitro. J Allergy Clin Immunol. 1987 Mar;79(3):496–504. doi: 10.1016/0091-6749(87)90368-x. [DOI] [PubMed] [Google Scholar]
- Andrews P. C., Krinsky N. I. The reductive cleavage of myeloperoxidase in half, producing enzymically active hemi-myeloperoxidase. J Biol Chem. 1981 May 10;256(9):4211–4218. [PubMed] [Google Scholar]
- Babcock G. T., Ingle R. T., Oertling W. A., Davis J. C., Averill B. A., Hulse C. L., Stufkens D. J., Bolscher B. G., Wever R. Raman characterization of human leukocyte myeloperoxidase and bovine spleen green haemoprotein. Insight into chromophore structure and evidence that the chromophores of myeloperoxidase are equivalent. Biochim Biophys Acta. 1985 Mar 22;828(1):58–66. doi: 10.1016/0167-4838(85)90009-3. [DOI] [PubMed] [Google Scholar]
- Bolscher B. G., Plat H., Wever R. Some properties of human eosinophil peroxidase, a comparison with other peroxidases. Biochim Biophys Acta. 1984 Jan 31;784(2-3):177–186. doi: 10.1016/0167-4838(84)90125-0. [DOI] [PubMed] [Google Scholar]
- Carlson M. G., Peterson C. G., Venge P. Human eosinophil peroxidase: purification and characterization. J Immunol. 1985 Mar;134(3):1875–1879. [PubMed] [Google Scholar]
- Griffin B. W. Mechanism of halide-stimulated activity of chloroperoxidase evidence for enzymatic formation of free hypohalous acid. Biochem Biophys Res Commun. 1983 Nov 15;116(3):873–879. doi: 10.1016/s0006-291x(83)80223-x. [DOI] [PubMed] [Google Scholar]
- Harman L. S., Carver D. K., Schreiber J., Mason R. P. One- and two-electron oxidation of reduced glutathione by peroxidases. J Biol Chem. 1986 Feb 5;261(4):1642–1648. [PubMed] [Google Scholar]
- Harman L. S., Mottley C., Mason R. P. Free radical metabolites of L-cysteine oxidation. J Biol Chem. 1984 May 10;259(9):5606–5611. [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Himmelhoch S. R., Evans W. H., Mage M. G., Peterson E. A. Purification of myeloperoxidases from the bone marrow of the guinea pig. Biochemistry. 1969 Mar;8(3):914–921. doi: 10.1021/bi00831a022. [DOI] [PubMed] [Google Scholar]
- Kimura S., Yamazaki I. Comparisons between hog intestinal peroxidase and bovine lactoperoxidase-compound I formation and inhibition by benzhydroxamic acid. Arch Biochem Biophys. 1979 Dec;198(2):580–588. doi: 10.1016/0003-9861(79)90534-4. [DOI] [PubMed] [Google Scholar]
- Lessler M. A. Oxygen electrode measurements in biochemical analysis. Methods Biochem Anal. 1969;17:1–29. doi: 10.1002/9780470110355.ch1. [DOI] [PubMed] [Google Scholar]
- MASON H. S. Mechanisms of oxygen metabolism. Adv Enzymol Relat Subj Biochem. 1957;19:79–233. doi: 10.1002/9780470122648.ch2. [DOI] [PubMed] [Google Scholar]
- MORRISON M., HAMILTON H. B., STOTZ E. The isolation and purification of lactoperoxidase by ion exchange chromatography. J Biol Chem. 1957 Oct;228(2):767–776. [PubMed] [Google Scholar]
- Migler R., DeChatelet L. R., Bass D. A. Human eosinophilic peroxidase: role in bactericidal activity. Blood. 1978 Mar;51(3):445–456. [PubMed] [Google Scholar]
- Morrison M., Schonbaum G. R. Peroxidase-catalyzed halogenation. Annu Rev Biochem. 1976;45:861–888. doi: 10.1146/annurev.bi.45.070176.004241. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Yamazaki I., Kotani T., Ohtaki S. Thyroid peroxidase selects the mechanism of either 1- or 2-electron oxidation of phenols, depending on their substituents. J Biol Chem. 1985 Nov 5;260(25):13546–13552. [PubMed] [Google Scholar]
- Nakamura M., Yamazaki I., Ohtaki S., Nakamura S. Characterization of one- and two-electron oxidations of glutathione coupled with lactoperoxidase and thyroid peroxidase reactions. J Biol Chem. 1986 Oct 25;261(30):13923–13927. [PubMed] [Google Scholar]
- Olsen J., Davis L. The oxidation of dithiothreitol by peroxidases and oxygen. Biochim Biophys Acta. 1976 Sep 14;445(2):324–329. doi: 10.1016/0005-2744(76)90086-3. [DOI] [PubMed] [Google Scholar]
- Ross D., Norbeck K., Moldéus P. The generation and subsequent fate of glutathionyl radicals in biological systems. J Biol Chem. 1985 Dec 5;260(28):15028–15032. [PubMed] [Google Scholar]
- Sawada Y., Yamazaki I. One-electron transfer reactions in biochemical systems. 8. Kinetic study of superoxide dismutase. Biochim Biophys Acta. 1973 Dec 19;327(2):257–265. doi: 10.1016/0005-2744(73)90408-7. [DOI] [PubMed] [Google Scholar]
- Smith A. M., Morrison W. L., Milham P. J. Oxidation of indole-3-acetic acid by peroxidase: involvement of reduced peroxidase and compound III with superoxide as a product. Biochemistry. 1982 Aug 31;21(18):4414–4419. doi: 10.1021/bi00261a034. [DOI] [PubMed] [Google Scholar]
- Svensson B. E., Domeij K., Lindvall S., Rydell G. Peroxidase and peroxidase-oxidase activities of isolated human myeloperoxidases. Biochem J. 1987 Mar 15;242(3):673–680. doi: 10.1042/bj2420673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson B. E., Lindvall S. Myeloperoxidase-oxidase oxidation of cysteamine. Biochem J. 1988 Jan 15;249(2):521–530. doi: 10.1042/bj2490521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson B. E. Myeloperoxidase oxidation states involved in myeloperoxidase-oxidase oxidation of thiols. Biochem J. 1988 Dec 15;256(3):751–755. doi: 10.1042/bj2560751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson B. E. Thiols as myeloperoxidase-oxidase substrates. Biochem J. 1988 Jul 15;253(2):441–449. doi: 10.1042/bj2530441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkall R. M., Tsan M. F. Oxidation of glutathione by the myeloperoxidase system. J Reticuloendothel Soc. 1982 Apr;31(4):353–360. [PubMed] [Google Scholar]
- Wefers H., Riechmann E., Sies H. Excited species generation in horseradish peroxidase-mediated oxidation of glutathione. J Free Radic Biol Med. 1985;1(4):311–318. doi: 10.1016/0748-5514(85)90137-0. [DOI] [PubMed] [Google Scholar]
- Wever R., Plat H., Hamers M. N. Human eosinophil peroxidase: a novel isolation procedure, spectral properties and chlorinating activity. FEBS Lett. 1981 Jan 26;123(2):327–331. doi: 10.1016/0014-5793(81)80320-1. [DOI] [PubMed] [Google Scholar]
- YAMAZAKI I., PIETTE L. H. THE MECHANISM OF AEROBIC OXIDASE REACTION CATALYZED BY PEROXIDASE. Biochim Biophys Acta. 1963 Sep 3;77:47–64. doi: 10.1016/0006-3002(63)90468-2. [DOI] [PubMed] [Google Scholar]
- Yamazaki I., Yokota K. Oxidation states of peroxidase. Mol Cell Biochem. 1973 Nov 15;2(1):39–52. doi: 10.1007/BF01738677. [DOI] [PubMed] [Google Scholar]
- Yokota K., Yamazaki I. Analysis and computer simulation of aerobic oxidation of reduced nicotinamide adenine dinucleotide catalyzed by horseradish peroxidase. Biochemistry. 1977 May 3;16(9):1913–1920. doi: 10.1021/bi00628a024. [DOI] [PubMed] [Google Scholar]


