Abstract
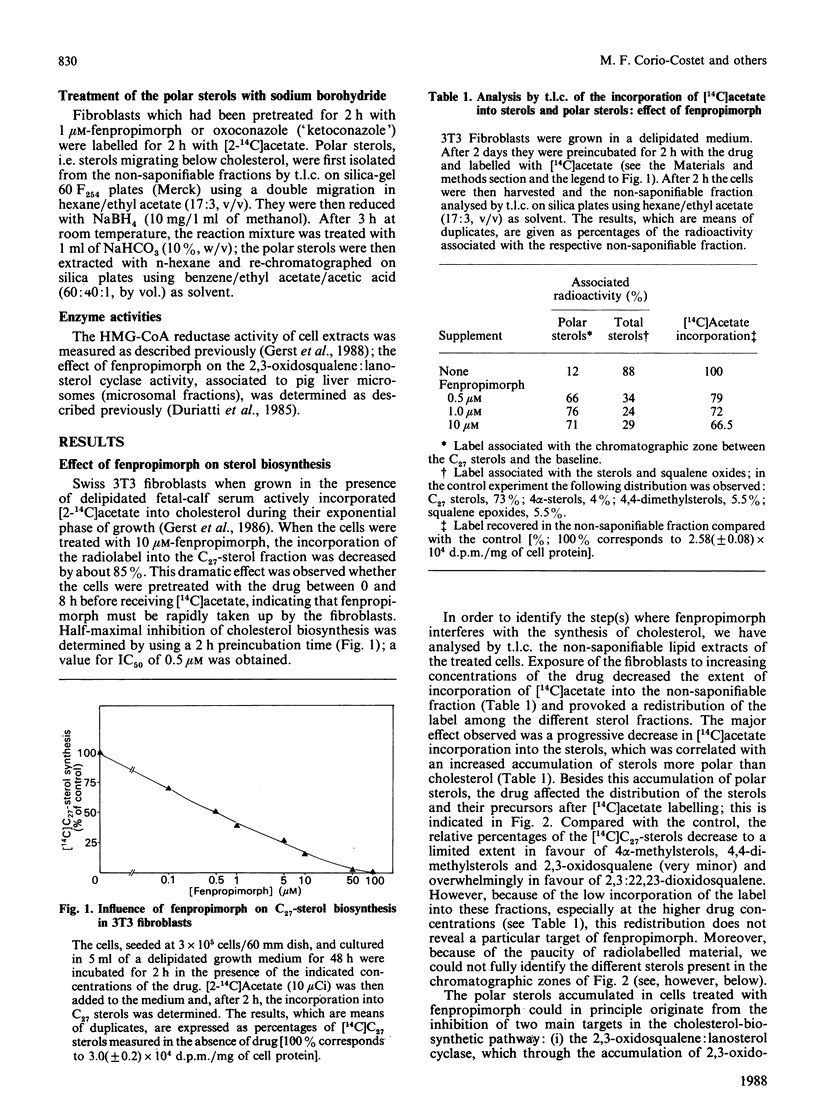
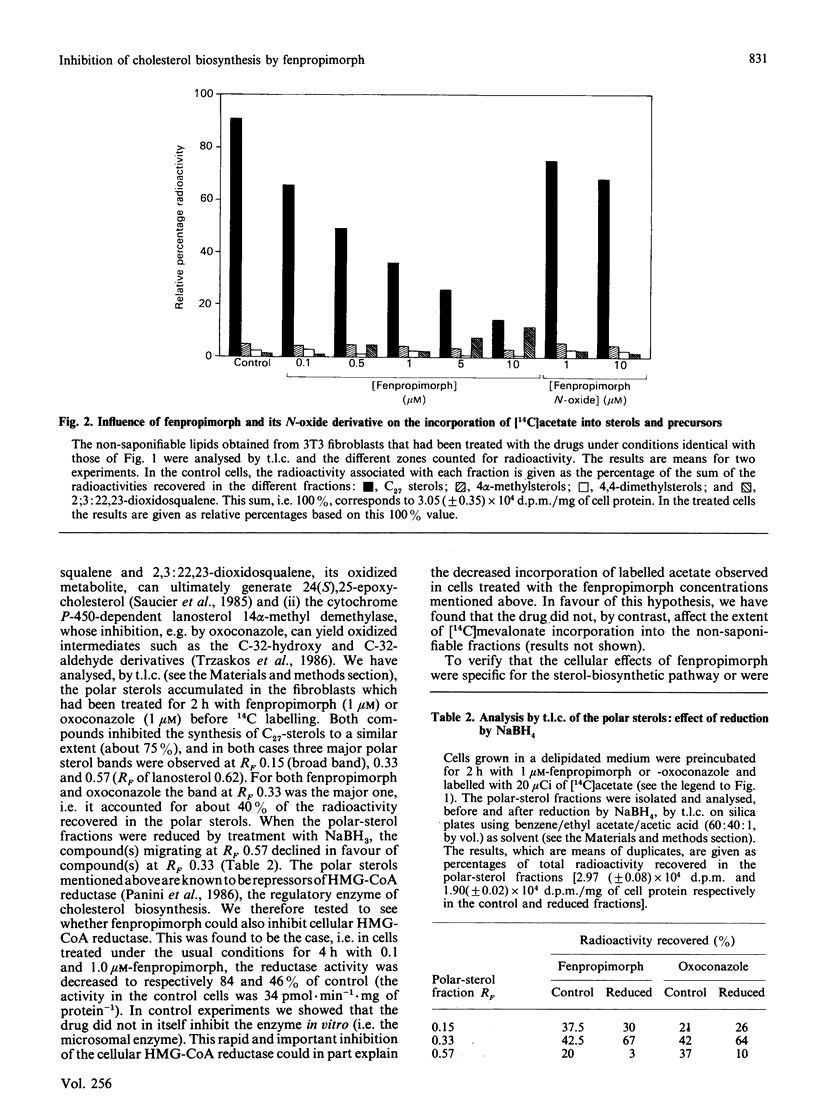
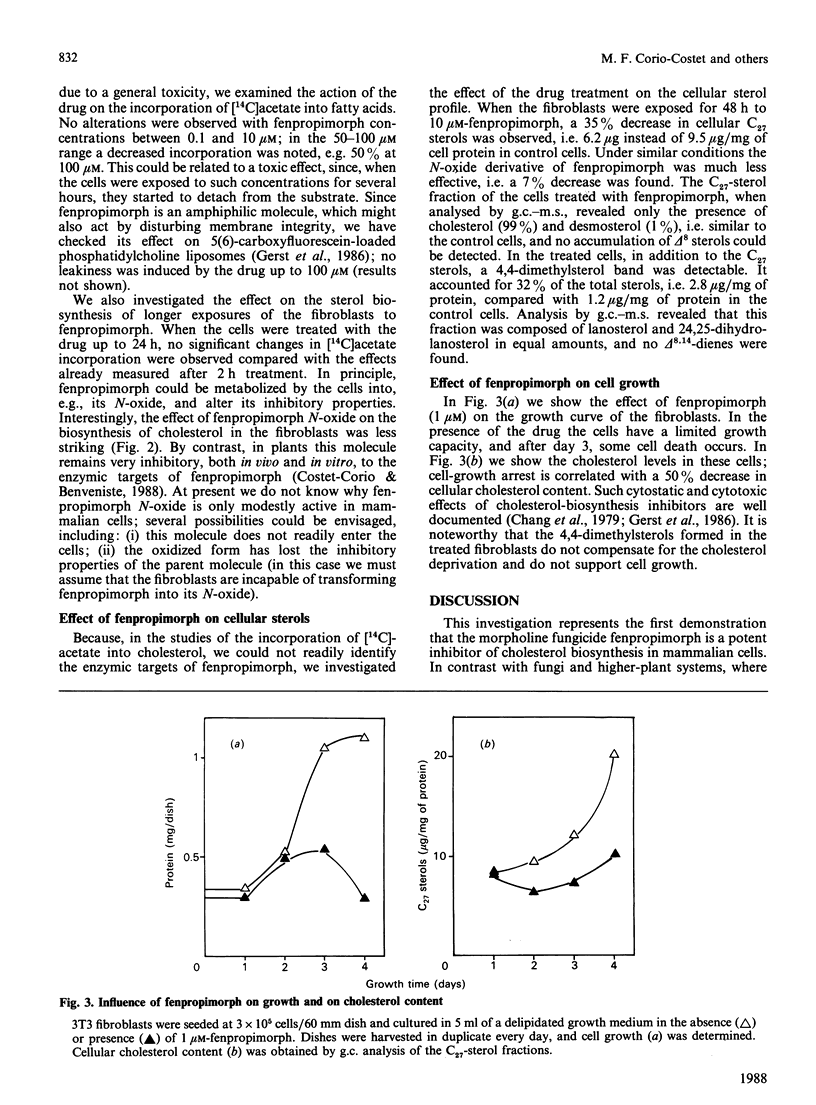
Fenpropimorph (N-[3-(p-t-butylphenyl)-2-methylpropyl]-cis-2,6-dimethylmorpholine), a morpholine fungicide known to be an inhibitor of sterol biosynthesis in fungi and in higher plants, was demonstrated to be an efficient inhibitor of cholesterol biosynthesis in cultured Swiss 3T3 fibroblasts. Treatment of the mammalian cells with fenpropimorph resulted in a dose-dependent inhibition of [14C]acetate incorporation into the C27 sterols [IC50 (concentration causing half-maximal inhibition) = 0.5 microM], which was accompanied by an accumulation of polar sterols and a decrease in cellular hydroxymethylglutaryl-CoA reductase activity. Exposure of the cells to the drug affected cell growth. Analysis of the sterols in the growth-arrested and in the pulse-labelled cells indicate that fenpropimorph has, in the sterol-biosynthetic pathway, target enzymes in mammalian cells different from those in the other phyla. Whereas in plants and fungi fenpropimorph mainly affects sterol isomerases and reductases, in the fibroblasts its main target seems to be the demethylation of lanosterol.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang T. Y., Schiavoni E. S., Jr, McCrae K. R., Nelson J. A., Spencer T. A. Inhibition of cholesterol biosynthesis in Chinese hamster ovary cells by 4,4,10 beta-trimethyl-trans-decal-3 beta-ol. A specific 2,3-oxidosqualene cyclase inhibitor. J Biol Chem. 1979 Nov 25;254(22):11258–11263. [PubMed] [Google Scholar]
- Costet M. F., El Achouri M., Charlet M., Lanot R., Benveniste P., Hoffmann J. A. Ecdysteroid biosynthesis and embryonic development are disturbed in insects (Locusta migratoria) reared on plant diet (Triticum sativum) with a selectively modified sterol profile. Proc Natl Acad Sci U S A. 1987 Feb;84(3):643–647. doi: 10.1073/pnas.84.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duriatti A., Bouvier-Nave P., Benveniste P., Schuber F., Delprino L., Balliano G., Cattel L. In vitro inhibition of animal and higher plants 2,3-oxidosqualene-sterol cyclases by 2-aza-2,3-dihydrosqualene and derivatives, and by other ammonium-containing molecules. Biochem Pharmacol. 1985 Aug 1;34(15):2765–2777. doi: 10.1016/0006-2952(85)90578-7. [DOI] [PubMed] [Google Scholar]
- Favata M. F., Trzaskos J. M., Chen H. W., Fischer R. T., Greenberg R. S. Modulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase by azole antimycotics requires lanosterol demethylation, but not 24,25-epoxylanosterol formation. J Biol Chem. 1987 Sep 5;262(25):12254–12260. [PubMed] [Google Scholar]
- Gerst N., Duriatti A., Schuber F., Taton M., Benveniste P., Rahier A. Potent inhibition of cholesterol biosynthesis in 3T3 fibroblasts by N-[(1,5,9)-trimethyldecyl]-4 alpha,10-dimethyl-8-aza-trans-decal-3 beta-ol, a new 2,3-oxidosqualene cyclase inhibitor. Biochem Pharmacol. 1988 May 15;37(10):1955–1964. doi: 10.1016/0006-2952(88)90542-4. [DOI] [PubMed] [Google Scholar]
- Gerst N., Schuber F., Viola F., Cattel L. Inhibition of cholesterol biosynthesis in 3T3 fibroblasts by 2-aza-2,3-dihydrosqualene, a rationally designed 2,3-oxidosqualene cyclase inhibitor. Biochem Pharmacol. 1986 Dec 1;35(23):4243–4250. doi: 10.1016/0006-2952(86)90702-1. [DOI] [PubMed] [Google Scholar]
- Kandutsch A. A., Saucier S. E. Prevention of cyclic and triton-induced increases in hydroxymethylglutaryl coenzyme A reductase and sterol synthesis by puromycin. J Biol Chem. 1969 May 10;244(9):2299–2305. [PubMed] [Google Scholar]
- Mercer E. I., Morris P. K., Baldwin B. C. Differences in the inhibitory effects of N-(1-n-dodecyl)-heterocycles on the 2,3-oxidosqualene lanosterol-cyclase of rat liver and yeast. Comp Biochem Physiol B. 1985;80(2):341–346. doi: 10.1016/0305-0491(85)90216-0. [DOI] [PubMed] [Google Scholar]
- Müller W., Schewe T. Das Systemfungizid Tridemorph als Hemmstoff der Atmungskette von Elektronentransportpartikeln aus Rinderherzmitochondrien. Acta Biol Med Ger. 1976;35(6):693–707. [PubMed] [Google Scholar]
- Panini S. R., Sexton R. C., Gupta A. K., Parish E. J., Chitrakorn S., Rudney H. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and cholesterol biosynthesis by oxylanosterols. J Lipid Res. 1986 Nov;27(11):1190–1204. [PubMed] [Google Scholar]
- Panini S. R., Sexton R. C., Rudney H. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase by oxysterol by-products of cholesterol biosynthesis. Possible mediators of low density lipoprotein action. J Biol Chem. 1984 Jun 25;259(12):7767–7771. [PubMed] [Google Scholar]
- Rudney H., Sexton R. C. Regulation of cholesterol biosynthesis. Annu Rev Nutr. 1986;6:245–272. doi: 10.1146/annurev.nu.06.070186.001333. [DOI] [PubMed] [Google Scholar]
- Ryder N. S., Dupont M. C. Inhibition of squalene epoxidase by allylamine antimycotic compounds. A comparative study of the fungal and mammalian enzymes. Biochem J. 1985 Sep 15;230(3):765–770. doi: 10.1042/bj2300765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucier S. E., Kandutsch A. A., Taylor F. R., Spencer T. A., Phirwa S., Gayen A. K. Identification of regulatory oxysterols, 24(S),25-epoxycholesterol and 25-hydroxycholesterol, in cultured fibroblasts. J Biol Chem. 1985 Nov 25;260(27):14571–14579. [PubMed] [Google Scholar]
- Trzaskos J. M., Favata M. F., Fischer R. T., Stam S. H. In situ accumulation of 3 beta-hydroxylanost-8-en-32-aldehyde in hepatocyte cultures. A putative regulator of 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity. J Biol Chem. 1987 Sep 5;262(25):12261–12268. [PubMed] [Google Scholar]
- Trzaskos J. M., Fischer R. T., Favata M. F. Mechanistic studies of lanosterol C-32 demethylation. Conditions which promote oxysterol intermediate accumulation during the demethylation process. J Biol Chem. 1986 Dec 25;261(36):16937–16942. [PubMed] [Google Scholar]
- Vanden Bossche H. Biochemical targets for antifungal azole derivatives: hypothesis on the mode of action. Curr Top Med Mycol. 1985;1:313–351. doi: 10.1007/978-1-4613-9547-8_12. [DOI] [PubMed] [Google Scholar]